Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Closest-Packing Water Monolayer Stably Intercalated in Phyllosilicate Minerals under High Pressure.
Langmuir ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.langmuir.9b03394 Meng Chen 1 , Huijun Zhou 1, 2 , Runliang Zhu 1 , Xiancai Lu 3 , Hongping He 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.langmuir.9b03394 Meng Chen 1 , Huijun Zhou 1, 2 , Runliang Zhu 1 , Xiancai Lu 3 , Hongping He 1, 2
Affiliation
|
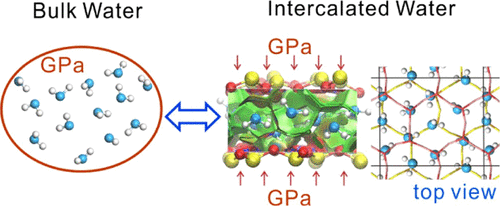
The directional hydrogen-bond (HB) network and nondirectional van der Waals (vdW) interactions make up the specificity of water. Directional HBs could construct an ice-like monolayer in hydrophobic confinement even in the ambient regime. Here, we report a water monolayer dominated by vdW interactions confined in a phyllosilicate interlayer under high pressure. Surprisingly, it was in a thermodynamically stable state coupled with bulk water at the same pressure (P) and temperature (T), as revealed by the thermodynamic integration approach on the basis of molecular dynamics (MD) simulations. Both classical and ab initio MD simulations showed water O atoms were stably trapped and exhibited an ordered hexagonal closest-packing arrangement, but OH bonds of water reoriented frequently and exhibited a specific two-stage reorientation relaxation. Strikingly, hydration in the interlayer under high pressure had no relevance with surface hydrophilicity rationalized by the HB forming ability, which, however, determines wetting in the ambient regime. Intercalated water molecules were trapped by vdW interactions, which shaped the closest-packing arrangement and made hydration energetically available. The high pressure-volume term largely drives hydration, as it compensates the entropy penalty which is restricted by a relatively lower temperature. This vdW water monolayer should be ubiquitous in the high pressure but low-temperature regime.
中文翻译:

在高压下稳定地插入到层状硅酸盐矿物中的最紧密堆积的水单层。
定向氢键(HB)网络和非定向范德华(vdW)相互作用构成了水的特异性。定向HBs甚至在环境条件下也可以在疏水限制下构建冰状单层。在这里,我们报道了在高压下,由单层硅酸盐层间的vdW相互作用控制的水单层。出乎意料的是,它处于热力学稳定状态,并且在相同的压力(P)和温度(T)下与大量水耦合,这是基于分子动力学(MD)模拟的热力学积分方法揭示的。经典的和从头算的MD模拟都显示水O原子被稳定地俘获并表现出有序的六边形最紧密堆积排列,但是水的OH键频繁地重新定向并表现出特定的两阶段重新定向弛豫。令人惊讶的是,在高压下,中间层中的水合作用与通过HB形成能力合理化的表面亲水性无关,然而,这决定了环境条件下的润湿性。嵌入的水分子被vdW相互作用捕获,这形成了最紧密的堆积结构,使水化能得到有效利用。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。形成最紧密的包装结构,使水合作用更加活跃。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。形成最紧密的包装结构,使水合作用更加活跃。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。
更新日期:2020-01-09
中文翻译:

在高压下稳定地插入到层状硅酸盐矿物中的最紧密堆积的水单层。
定向氢键(HB)网络和非定向范德华(vdW)相互作用构成了水的特异性。定向HBs甚至在环境条件下也可以在疏水限制下构建冰状单层。在这里,我们报道了在高压下,由单层硅酸盐层间的vdW相互作用控制的水单层。出乎意料的是,它处于热力学稳定状态,并且在相同的压力(P)和温度(T)下与大量水耦合,这是基于分子动力学(MD)模拟的热力学积分方法揭示的。经典的和从头算的MD模拟都显示水O原子被稳定地俘获并表现出有序的六边形最紧密堆积排列,但是水的OH键频繁地重新定向并表现出特定的两阶段重新定向弛豫。令人惊讶的是,在高压下,中间层中的水合作用与通过HB形成能力合理化的表面亲水性无关,然而,这决定了环境条件下的润湿性。嵌入的水分子被vdW相互作用捕获,这形成了最紧密的堆积结构,使水化能得到有效利用。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。形成最紧密的包装结构,使水合作用更加活跃。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。形成最紧密的包装结构,使水合作用更加活跃。高压-体积项在很大程度上驱动水合作用,因为它补偿了由相对较低的温度所限制的熵损失。这种vdW水单层在高压但低温条件下应无处不在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号