当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Spectroscopy of 2-Phenyl-1,3,2-benzodioxaborole and Its Derivatives: Important Building Blocks of Covalent Organic Frameworks.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jpca.9b09476 Cara Savino 1 , Roberta P Ryan 1 , Joseph L Knee 1 , Carlos A Jimenez-Hoyos 1 , Brian H Northrop 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jpca.9b09476 Cara Savino 1 , Roberta P Ryan 1 , Joseph L Knee 1 , Carlos A Jimenez-Hoyos 1 , Brian H Northrop 1
Affiliation
|
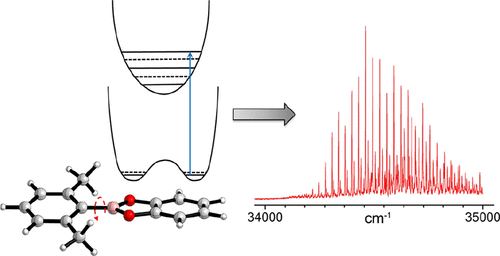
Aryl boronate esters, such as 2-phenyl-1,3,2-benzodioxaborole (1), are important components in the formation of a variety of covalent organic frameworks. The addition of substituents on the aromatic rings of aryl boronate esters has the potential to modify the structure, reactivity, and electronic properties of the resulting materials, and so, it is useful to understand at a more fundamental level the properties of these important compounds. Experimental measurements and computational investigations are presented herein that provide insight regarding the structural and electronic properties of parent aryl boronate ester 1 as well as three substituted derivatives: 2-(o-tolyl)-1,3,2-benzodioxaborole (2), 2-(2,6-dimethylphenyl)-1,3,2-benzodioxaborole (3), and 2-(4-(tert-butyl)phenyl)-1,3,2-benzodioxaborole (4). Electronic spectroscopy combined with excited-state calculations reveal two closely spaced electronic states, S1 and S2, which appear to have excitation primarily localized on the aromatic system of the phenyl substituent or the catecholborane moiety, respectively. Interestingly, the ortho-dimethyl derivative (3) shows a significantly red-shifted electronic origin with an extensive vibronic progression of a low-frequency torsional motion about the C-B bond. Franck-Condon calculations on the ab initio determined ground- and excited-state potentials very accurately reproduce this spectrum, confirming the nonplanar ground state of this compound.
中文翻译:

2-苯基-1,3,2-苯并二恶唑硼烷及其衍生物的电子光谱:共价有机框架的重要组成部分。
芳基硼酸酯,例如2-苯基-1,3,2-苯并二恶唑硼酸酯(1),是形成各种共价有机骨架的重要组分。在芳基硼酸酯的芳环上添加取代基具有改变所得材料的结构,反应性和电子性质的潜力,因此,在更基本的水平上理解这些重要化合物的性质是有用的。本文介绍了实验测量和计算研究,可深入了解母体芳基硼酸酯1以及三种取代的衍生物的结构和电子性能:2-(邻甲苯基)-1,3,2-苯并二恶杂硼酸酯(2),2 -(2,6-二甲基苯基)-1,3,2-苯并二氧杂硼酸酯(3)和2-(4-(叔丁基)苯基)-1,3,2-苯并二氧杂硼酸酯(4)。电子光谱结合激发态计算揭示了两个紧密排列的电子态S1和S2,它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。
更新日期:2020-01-10
中文翻译:

2-苯基-1,3,2-苯并二恶唑硼烷及其衍生物的电子光谱:共价有机框架的重要组成部分。
芳基硼酸酯,例如2-苯基-1,3,2-苯并二恶唑硼酸酯(1),是形成各种共价有机骨架的重要组分。在芳基硼酸酯的芳环上添加取代基具有改变所得材料的结构,反应性和电子性质的潜力,因此,在更基本的水平上理解这些重要化合物的性质是有用的。本文介绍了实验测量和计算研究,可深入了解母体芳基硼酸酯1以及三种取代的衍生物的结构和电子性能:2-(邻甲苯基)-1,3,2-苯并二恶杂硼酸酯(2),2 -(2,6-二甲基苯基)-1,3,2-苯并二氧杂硼酸酯(3)和2-(4-(叔丁基)苯基)-1,3,2-苯并二氧杂硼酸酯(4)。电子光谱结合激发态计算揭示了两个紧密排列的电子态S1和S2,它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。它们似乎分别主要位于苯基取代基或儿茶酚硼烷部分的芳族体系上。有趣的是,邻二甲基衍生物(3)显示出明显的红移电子起源,并且围绕CB键的低频扭转运动发生了广泛的振动。从头算起,由弗兰克-康登(Franck-Condon)计算得出的基态和激发态电势非常准确地重现了该光谱,从而确认了该化合物的非平面基态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号