当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Cross-Dehydrogenative C-O Coupling of Carbonyl Compounds with N-Hydroxyimides: Unexpected Selective Behavior of Highly Reactive Free Radicals at an Elevated Temperature.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.joc.9b02656 Igor B Krylov 1 , Elena R Lopat'eva 1, 2 , Alexander S Budnikov 1, 2 , Gennady I Nikishin 1 , Alexander O Terent'ev 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.joc.9b02656 Igor B Krylov 1 , Elena R Lopat'eva 1, 2 , Alexander S Budnikov 1, 2 , Gennady I Nikishin 1 , Alexander O Terent'ev 1
Affiliation
|
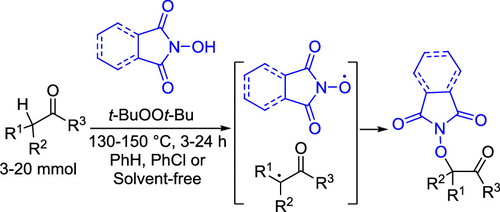
Cross-dehydrogenative C-O coupling of N-hydroxyimides with ketones, esters, and carboxylic acids was achieved employing the di-tert-butyl peroxide as a source of free radicals and a dehydrogenating agent. The proposed method is experimentally simple and demonstrates the outstanding efficiency for the challenging CH substrates, such as unactivated esters and carboxylic acids. It was shown that N-hydroxyphthalimide drastically affects the oxidative properties of t-BuOOt-Bu by intercepting the t-BuO• radicals with the formation of phthalimide-N-oxyl radicals, a species responsible for both hydrogen atom abstraction from the CH reagent and the selective formation of the C-O coupling product by selective radical cross-recombination. The practical applicability of the developed method was exemplified by the single-stage synthesis of commercial reagent (known as Baran aminating reagent precursor) from isobutyric acid and N-hydroxysuccinimide, whereas in the standard synthetic approach, four stages are necessary.
中文翻译:

羰基化合物与N-羟基酰亚胺的无金属交叉脱氢CO偶联:高反应性自由基在高温下的意外选择行为。
N-羟基酰亚胺与酮,酯和羧酸的交叉脱氢CO偶联是通过使用二叔丁基过氧化物作为自由基源和脱氢剂来实现的。所提出的方法在实验上很简单,并且证明了对于具有挑战性的CH底物(例如未活化的酯和羧酸)具有出色的效率。结果表明,N-羟基邻苯二甲酰亚胺通过拦截t-BuO•自由基而形成邻苯二甲酰亚胺-N-氧基自由基,从而极大地影响t-BuOOt-Bu的氧化性能。通过选择性自由基交叉重组选择性形成CO偶联产物。
更新日期:2020-01-14
中文翻译:

羰基化合物与N-羟基酰亚胺的无金属交叉脱氢CO偶联:高反应性自由基在高温下的意外选择行为。
N-羟基酰亚胺与酮,酯和羧酸的交叉脱氢CO偶联是通过使用二叔丁基过氧化物作为自由基源和脱氢剂来实现的。所提出的方法在实验上很简单,并且证明了对于具有挑战性的CH底物(例如未活化的酯和羧酸)具有出色的效率。结果表明,N-羟基邻苯二甲酰亚胺通过拦截t-BuO•自由基而形成邻苯二甲酰亚胺-N-氧基自由基,从而极大地影响t-BuOOt-Bu的氧化性能。通过选择性自由基交叉重组选择性形成CO偶联产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号