当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of an Unusual Tetracyclic Deoxyguanosine Adduct: Implications for Frameshift Mutagenicity of ortho-Cyano Nitroanilines.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.chemrestox.9b00411 Trevor W Manning , M Sameer Al-Abdul-Wahid , Richard A Manderville , P David Josephy , Ryan W Kung 1 , Stacey D Wetmore 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.chemrestox.9b00411 Trevor W Manning , M Sameer Al-Abdul-Wahid , Richard A Manderville , P David Josephy , Ryan W Kung 1 , Stacey D Wetmore 1
Affiliation
|
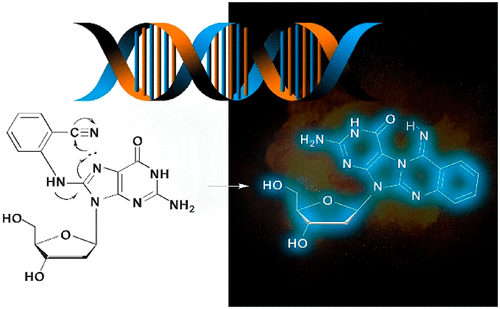
Nitroaromatic compounds represent a major class of industrial chemicals that are also found in nature. Polycyclic derivatives are regarded as potent mutagens and carcinogens following bioactivation to produce nitrenium electrophiles that covalently modify DNA to afford N-linked C8-2'-deoxyguanosine (C8-dG) lesions that can induce frameshift mutations, especially in CpG repeat sequences. In contrast, their monocyclic counterparts typically exhibit weak mutagenicity or a lack thereof, despite also undergoing bioactivation to afford N-linked C8-dG adducts. Recently, it has been reported that cyano substitution can greatly increase the mutagenicity of nitroaniline derivatives that are components of azo dyes. The basis of this "cyano effect" may be rooted in the formation of a novel polycyclic adduct arising from initial formation of the N-linked C8-dG adduct followed by a cyclization process involving N7 of dG and the ortho-CN group of the attached C8-aryl moiety to generate a quinazolinimine ring as part of a fused tetracyclic C8,N7-dG adduct structure. The present work structurally characterizes this novel cyclic adduct using a combination of optical spectroscopies, NMR analysis, density functional theory (DFT) calculations, and molecular dynamics (MD) simulations. Our data indicate that this highly fluorescent cyclic adduct adopts the promutagenic syn conformation and can stabilize the slipped mutagenic intermediate (SMI) within the CpG repeat of the NarI sequence, which is a hotspot for frameshift mutagenesis mediated by polycyclic N-linked C8-dG adducts. In contrast, the open para-CN (4-aminobenzontrile-derived) N-linked C8-dG adduct is less likely to disrupt the canonical B-form. Together, our results provide a rationale for the potent mutagenicity of cyano-substituted nitroaniline derivatives recently reported in frameshift-sensitive tester strains.
中文翻译:

异常的四环脱氧鸟苷加合物的结构:对邻氰基硝基苯胺的移码致突变性的影响。
硝基芳族化合物代表了自然界中也发现的一类主要的工业化学物质。多环衍生物被生物激活后产生有效的诱变剂和致癌物,这些亲电子可共价修饰DNA以提供N-连接的C8-2'-脱氧鸟苷(C8-dG)损伤,可诱导移码突变,尤其是在CpG重复序列中。相反,它们的单环对应物通常表现出弱的致突变性或缺乏诱变性,尽管还经历了生物活化以提供N-连接的C8-dG加合物。近来,已经报道氰基取代可以极大地增加作为偶氮染料的组分的硝基苯胺衍生物的诱变性。这种“氰基效应”的基础 可能根源于新的多环加合物的形成,该新的多环加合物的形成是由N-连接的C8-dG加合物的初始形成,随后涉及dG的N7和连接的C8-芳基部分的邻-CN基团的环化过程产生的喹唑啉亚胺环作为稠合四环C8,N7-dG加合物结构的一部分。本工作使用光学光谱学,NMR分析,密度泛函理论(DFT)计算和分子动力学(MD)模拟的组合在结构上表征了这种新型环状加合物。我们的数据表明,这种高度荧光的环状加合物采用了诱变的顺式构象,可以稳定NarI序列CpG重复序列内的致突变诱变中间体(SMI),这是由多环N-连接的C8-dG加合物介导的移码诱变的热点。相比之下,开放的对-CN(源自4-氨基苯甲腈)的N-连接的C8-dG加合物不太可能破坏规范的B型。总之,我们的结果为移码敏感测试菌株中最近报道的氰基取代的硝基苯胺衍生物的强致突变性提供了理论依据。
更新日期:2020-01-10
中文翻译:

异常的四环脱氧鸟苷加合物的结构:对邻氰基硝基苯胺的移码致突变性的影响。
硝基芳族化合物代表了自然界中也发现的一类主要的工业化学物质。多环衍生物被生物激活后产生有效的诱变剂和致癌物,这些亲电子可共价修饰DNA以提供N-连接的C8-2'-脱氧鸟苷(C8-dG)损伤,可诱导移码突变,尤其是在CpG重复序列中。相反,它们的单环对应物通常表现出弱的致突变性或缺乏诱变性,尽管还经历了生物活化以提供N-连接的C8-dG加合物。近来,已经报道氰基取代可以极大地增加作为偶氮染料的组分的硝基苯胺衍生物的诱变性。这种“氰基效应”的基础 可能根源于新的多环加合物的形成,该新的多环加合物的形成是由N-连接的C8-dG加合物的初始形成,随后涉及dG的N7和连接的C8-芳基部分的邻-CN基团的环化过程产生的喹唑啉亚胺环作为稠合四环C8,N7-dG加合物结构的一部分。本工作使用光学光谱学,NMR分析,密度泛函理论(DFT)计算和分子动力学(MD)模拟的组合在结构上表征了这种新型环状加合物。我们的数据表明,这种高度荧光的环状加合物采用了诱变的顺式构象,可以稳定NarI序列CpG重复序列内的致突变诱变中间体(SMI),这是由多环N-连接的C8-dG加合物介导的移码诱变的热点。相比之下,开放的对-CN(源自4-氨基苯甲腈)的N-连接的C8-dG加合物不太可能破坏规范的B型。总之,我们的结果为移码敏感测试菌株中最近报道的氰基取代的硝基苯胺衍生物的强致突变性提供了理论依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号