当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A dienamine-mediated deconjugative addition/cyclization cascade of γ,γ-disubstituted enals with carboxylic acid-activated enones: a rapid access to highly functionalized γ-lactones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019/12/30 , DOI: 10.1039/c9qo01367a Wei Jiang 1, 2, 3, 4, 5 , Jia Zhou 5, 6, 7, 8, 9 , Ai-Jun Ma 1, 2, 3, 4, 5 , Dongli Li 1, 2, 3, 4, 5 , Yan-Yan Ma 1, 2, 3, 4, 5 , Deng-Gao Zhao 1, 2, 3, 4, 5 , Si-Hua Hou 5, 6, 7, 8, 9 , Jun-Bing Lin 5, 10, 11, 12, 13 , Shu-Yu Zhang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019/12/30 , DOI: 10.1039/c9qo01367a Wei Jiang 1, 2, 3, 4, 5 , Jia Zhou 5, 6, 7, 8, 9 , Ai-Jun Ma 1, 2, 3, 4, 5 , Dongli Li 1, 2, 3, 4, 5 , Yan-Yan Ma 1, 2, 3, 4, 5 , Deng-Gao Zhao 1, 2, 3, 4, 5 , Si-Hua Hou 5, 6, 7, 8, 9 , Jun-Bing Lin 5, 10, 11, 12, 13 , Shu-Yu Zhang 1, 2, 3, 4, 5
Affiliation
|
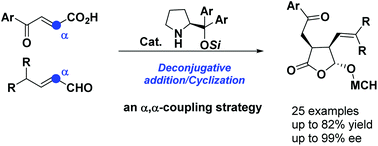
An aminocatalytic deconjugative addition/cyclization cascade of γ,γ-disubstituted enals with carboxylic acid-activated enones was realized. The in situ generated dienamine species was regioselectively trapped at the α position by carboxylic acid-activated enones, leading to highly functionalized γ-lactones with excellent enantioselectivities. Further synthetic elaboration of the products allows diverse synthesis of a set of complex chiral targets.
中文翻译:

γ,γ-二取代的烯醛与羧酸活化的烯酮的二烯胺介导的去共轭加成/环化级联反应:快速获得高度官能化的γ-内酯
实现了γ,γ-二取代烯醛与羧酸活化的烯酮的氨基催化去共轭加成/环化级联反应。该原位生成的氨醇二烯胺物种区域选择性地捕获在通过羧酸活化烯酮α位,导致高度官能γ内酯具有优异的对映选择性。产物的进一步合成精制允许一组复杂的手性靶标的多样化合成。
更新日期:2020-02-13
中文翻译:

γ,γ-二取代的烯醛与羧酸活化的烯酮的二烯胺介导的去共轭加成/环化级联反应:快速获得高度官能化的γ-内酯
实现了γ,γ-二取代烯醛与羧酸活化的烯酮的氨基催化去共轭加成/环化级联反应。该原位生成的氨醇二烯胺物种区域选择性地捕获在通过羧酸活化烯酮α位,导致高度官能γ内酯具有优异的对映选择性。产物的进一步合成精制允许一组复杂的手性靶标的多样化合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号