当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
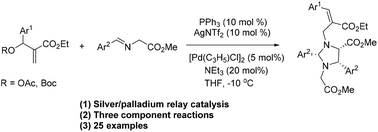
Silver/palladium relay catalyzed 1,3-dipole annulation/allylation reactions to access fully substituted allyl imidazolidines.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1039/c9ob02633a Ruiping Han 1 , Yue Ding 1 , Xueke Jin 1 , Er-Qing Li 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1039/c9ob02633a Ruiping Han 1 , Yue Ding 1 , Xueke Jin 1 , Er-Qing Li 1
Affiliation
|
A silver/palladium relay catalyzed 1,3-dipole annulation/allylation reaction of iminoesters and Baylis-Hillman acetates for the construction of fully substituted allyl imidazolidines is reported. The reaction of both iminoesters and Baylis-Hillman acetates affords the fully substituted allyl imidazolidines in high yields and regioselectivities. The three component reaction is triggered by silver-catalyzed 1,3-dipole annulation, followed by the sequential palladium catalyzed allylation. Mechanistic studies reveal that the dual catalytic system plays a key role in the reaction. The developed methodology provides straightforward access to allyl imidazolidines under simple reaction conditions.
中文翻译:

银/钯中继催化1,3-偶极环化/烯丙基化反应,以得到完全取代的烯丙基咪唑烷。
报道了银/钯中继催化的亚氨基酸酯和Baylis-Hillman乙酸酯的1,3-偶极环化/烯丙基化反应,用于构建完全取代的烯丙基咪唑烷。亚氨基酯和Baylis-Hillman乙酸酯的反应均以高收率和区域选择性提供了完全取代的烯丙基咪唑烷。银催化的1,3-偶极环化反应触发三组分反应,然后依次进行钯催化的烯丙基化反应。机理研究表明,双重催化体系在反应中起关键作用。开发的方法可在简单的反应条件下直接获得烯丙基咪唑烷。
更新日期:2020-02-10
中文翻译:

银/钯中继催化1,3-偶极环化/烯丙基化反应,以得到完全取代的烯丙基咪唑烷。
报道了银/钯中继催化的亚氨基酸酯和Baylis-Hillman乙酸酯的1,3-偶极环化/烯丙基化反应,用于构建完全取代的烯丙基咪唑烷。亚氨基酯和Baylis-Hillman乙酸酯的反应均以高收率和区域选择性提供了完全取代的烯丙基咪唑烷。银催化的1,3-偶极环化反应触发三组分反应,然后依次进行钯催化的烯丙基化反应。机理研究表明,双重催化体系在反应中起关键作用。开发的方法可在简单的反应条件下直接获得烯丙基咪唑烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号