当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jconrel.2019.12.049 D Christopher Radford 1 , Jiyuan Yang 2 , Mai C Doan 1 , Lian Li 2 , Andrew S Dixon 2 , Shawn C Owen 2 , Jindřich Kopeček 3
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jconrel.2019.12.049 D Christopher Radford 1 , Jiyuan Yang 2 , Mai C Doan 1 , Lian Li 2 , Andrew S Dixon 2 , Shawn C Owen 2 , Jindřich Kopeček 3
Affiliation
|
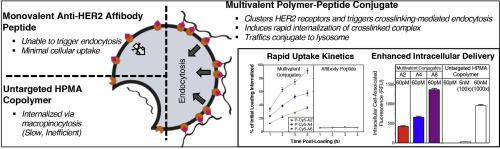
Incorporating targeting moieties that recognize cancer-specific cellular markers can enhance specificity of anticancer nanomedicines. The HER2 receptor is overexpressed on numerous cancers, making it an attractive target. However, unlike many receptors that trigger endocytosis upon ligand binding, HER2 is an internalization-resistant receptor. As most chemotherapeutics act on intracellular targets, this presents a significant challenge for exploiting HER2 overexpression for improved tumor killing. However, hyper-crosslinking of HER2 has been shown to override the receptor's native behavior and trigger internalization. This research co-opts this crosslinking-mediated internalization for efficient intracellular delivery of an anticancer nanomedicine - specifically a HPMA copolymer-based drug delivery system. This polymeric carrier was conjugated with a small (7 kDa) HER2-binding affibody peptide to produce a panel of polymer-affibody conjugates with valences from 2 to 10 peptides per polymer chain. The effect of valence on surface binding and uptake was evaluated separately. All conjugates demonstrated similar (nanomolar) binding affinity towards HER2-positive ovarian carcinoma cells, but higher-valence conjugates induced more rapid endocytosis, with over 90% of the surface-bound conjugate internalized within 4 h. Furthermore, this enhancement was sensitive to crowding - high surface loading reduced conjugates' ability to crosslink receptors. Collectively, this evidence strongly supports a crosslinking-mediated endocytosis mechanism. Lead candidates from this panel achieved high intracellular delivery even at picomolar treatment concentrations; untargeted HPMA copolymers required 1000-fold higher treatment concentrations to achieve similar levels of intracellular accumulation. This increased intracellular delivery also translated to a more potent nanomedicine against HER2-positive cells; incorporation of the chemotherapeutic paclitaxel into this targeted carrier enhanced cytotoxicity over untargeted polymer-drug conjugate.
中文翻译:

多价HER2结合聚合物共轭物有助于快速内吞并增强细胞内药物的递送。
结合识别癌症特异性细胞标志物的靶向部分可以增强抗癌纳米药物的特异性。HER2受体在多种癌症中过表达,使其成为有吸引力的靶标。但是,与许多受体在配体结合后触发内吞作用的受体不同,HER2是一种抗内在化的受体。由于大多数化学治疗剂作用于细胞内靶标,这对于利用HER2过表达来改善肿瘤杀伤力提出了重大挑战。但是,已证明HER2的超交联作用会取代受体的天然行为并触发内在化。这项研究选择了这种交联介导的内在化作用,以有效地在细胞内递送抗癌纳米药物-特别是基于HPMA共聚物的药物递送系统。将该聚合物载体与小的(7 kDa)HER2结合亲和体肽缀合,以产生一组聚合物-亲和体缀合物,每个聚合物链的化合价为2至10个肽。化合价对表面结合和吸收的影响分别进行了评估。所有结合物均表现出对HER2阳性卵巢癌细胞相似(纳摩尔)的结合亲和力,但价更高的结合物诱导更快的内吞作用,超过90%的表面结合结合物在4 h内被内化。此外,这种增强对拥挤敏感-高表面负荷降低了偶联物交联受体的能力。总体而言,该证据强烈支持交联介导的内吞作用机制。该小组的主要候选人即使在皮摩尔处理浓度下也能实现高细胞内递送;未靶向的HPMA共聚物需要高1000倍的处理浓度才能达到相似的细胞内积累水平。这种增加的细胞内传递也转化为针对HER2阳性细胞的更有效的纳米药物。与未靶向的聚合物-药物缀合物相比,将化疗紫杉醇掺入该靶向的载体增强了细胞毒性。
更新日期:2019-12-30
中文翻译:

多价HER2结合聚合物共轭物有助于快速内吞并增强细胞内药物的递送。
结合识别癌症特异性细胞标志物的靶向部分可以增强抗癌纳米药物的特异性。HER2受体在多种癌症中过表达,使其成为有吸引力的靶标。但是,与许多受体在配体结合后触发内吞作用的受体不同,HER2是一种抗内在化的受体。由于大多数化学治疗剂作用于细胞内靶标,这对于利用HER2过表达来改善肿瘤杀伤力提出了重大挑战。但是,已证明HER2的超交联作用会取代受体的天然行为并触发内在化。这项研究选择了这种交联介导的内在化作用,以有效地在细胞内递送抗癌纳米药物-特别是基于HPMA共聚物的药物递送系统。将该聚合物载体与小的(7 kDa)HER2结合亲和体肽缀合,以产生一组聚合物-亲和体缀合物,每个聚合物链的化合价为2至10个肽。化合价对表面结合和吸收的影响分别进行了评估。所有结合物均表现出对HER2阳性卵巢癌细胞相似(纳摩尔)的结合亲和力,但价更高的结合物诱导更快的内吞作用,超过90%的表面结合结合物在4 h内被内化。此外,这种增强对拥挤敏感-高表面负荷降低了偶联物交联受体的能力。总体而言,该证据强烈支持交联介导的内吞作用机制。该小组的主要候选人即使在皮摩尔处理浓度下也能实现高细胞内递送;未靶向的HPMA共聚物需要高1000倍的处理浓度才能达到相似的细胞内积累水平。这种增加的细胞内传递也转化为针对HER2阳性细胞的更有效的纳米药物。与未靶向的聚合物-药物缀合物相比,将化疗紫杉醇掺入该靶向的载体增强了细胞毒性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号