当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jconrel.2019.12.048 Xin Cheng 1 , Gu Cheng 2 , Xin Xing 1 , Chengcheng Yin 1 , Yuet Cheng 1 , Xue Zhou 3 , Shan Jiang 4 , Fenghua Tao 5 , Hongbing Deng 6 , Zubing Li 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.jconrel.2019.12.048 Xin Cheng 1 , Gu Cheng 2 , Xin Xing 1 , Chengcheng Yin 1 , Yuet Cheng 1 , Xue Zhou 3 , Shan Jiang 4 , Fenghua Tao 5 , Hongbing Deng 6 , Zubing Li 1
Affiliation
|
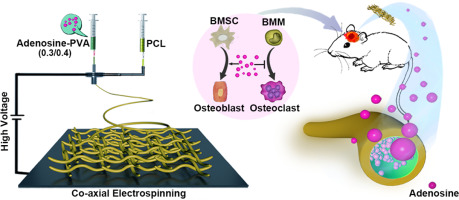
Adenosine (Ade) has been identified to stimulate bone formation. However, the use of Ade is severely limited by the accompanying side effects and its very short half-life in vivo. This study aimed to fabricate an efficient drug-delivery system to reduce the undesirable side effects and enable the clinical application of Ade for treating large bone defects. The fabricated poly(ε-caprolactone) (PCL)/Ade-polyvinyl alcohol (PVA)(0.3/0.4) nanofibrous mats with 0.3:0.4 (w/w) ratio of Ade and PVA showed a sustained and controlled release of Ade and facilitated the osteogenic differentiation of bone mesenchymal progenitor cells (BMSCs). A larger amount of newly formed bone was observed in vivo in the cranial defects of the PCL/Ade-PVA(0.3/0.4) group compared with those of the non-loaded PCL/PVA nanofibrous mats at 4 and 8 weeks after surgery. Moreover, it is the first time to confirm that Ade mediates the osteogenesis of rat BMSCs through the STAT3 signaling pathway and restrains the osteoclastogenesis of rat bone-marrow macrophages (BMMs). These results suggested that this coaxial drug-delivery system loaded with Ade provided a promising and clinically relevant platform to controlled-release Ade and address large bone defects.
中文翻译:

从核-壳纳米纤维中控制释放腺苷,以通过STAT3信号通路促进骨再生。
腺苷(Ade)已被确认可以刺激骨骼形成。然而,Ade的使用受到伴随的副作用及其在体内非常短的半衰期的严重限制。这项研究旨在制造一种有效的药物输送系统,以减少不良副作用,并使Ade的临床应用能够治疗大骨缺损。制备的Ade和PVA比例为0.3:0.4(w / w)的聚(ε-己内酯)(PCL)/ Ade-聚乙烯醇(PVA)(0.3 / 0.4)纳米纤维毡表现出Ade的持续释放和受控释放骨髓间充质祖细胞(BMSCs)的成骨分化。与未加载的PCL / PVA纳米纤维垫在手术后4周和8周相比,在PCL / Ade-PVA(0.3 / 0.4)组的颅骨缺损中体内观察到大量新形成的骨头。而且,这是首次证实Ade通过STAT3信号通路介导大鼠BMSC的成骨作用,并抑制大鼠骨髓巨噬细胞(BMM)的破骨细胞生成。这些结果表明,这种载有Ade的同轴药物输送系统为控释Ade和解决大骨缺损提供了一个有前途且与临床相关的平台。
更新日期:2019-12-30
中文翻译:

从核-壳纳米纤维中控制释放腺苷,以通过STAT3信号通路促进骨再生。
腺苷(Ade)已被确认可以刺激骨骼形成。然而,Ade的使用受到伴随的副作用及其在体内非常短的半衰期的严重限制。这项研究旨在制造一种有效的药物输送系统,以减少不良副作用,并使Ade的临床应用能够治疗大骨缺损。制备的Ade和PVA比例为0.3:0.4(w / w)的聚(ε-己内酯)(PCL)/ Ade-聚乙烯醇(PVA)(0.3 / 0.4)纳米纤维毡表现出Ade的持续释放和受控释放骨髓间充质祖细胞(BMSCs)的成骨分化。与未加载的PCL / PVA纳米纤维垫在手术后4周和8周相比,在PCL / Ade-PVA(0.3 / 0.4)组的颅骨缺损中体内观察到大量新形成的骨头。而且,这是首次证实Ade通过STAT3信号通路介导大鼠BMSC的成骨作用,并抑制大鼠骨髓巨噬细胞(BMM)的破骨细胞生成。这些结果表明,这种载有Ade的同轴药物输送系统为控释Ade和解决大骨缺损提供了一个有前途且与临床相关的平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号