当前位置:
X-MOL 学术
›
Am. J. Transplant.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rejection of xenogeneic porcine islets in humanized mice is characterized by graft-infiltrating Th17 cells and activated B cells.
American Journal of Transplantation ( IF 8.9 ) Pub Date : 2019-12-28 , DOI: 10.1111/ajt.15763 Frances T Lee 1 , Anil Dangi 2 , Sahil Shah 3 , Melanie Burnette 2 , Yong-Guang Yang 4 , Allan D Kirk 5 , Bernhard J Hering 6 , Stephen D Miller 7 , Xunrong Luo 2, 5
American Journal of Transplantation ( IF 8.9 ) Pub Date : 2019-12-28 , DOI: 10.1111/ajt.15763 Frances T Lee 1 , Anil Dangi 2 , Sahil Shah 3 , Melanie Burnette 2 , Yong-Guang Yang 4 , Allan D Kirk 5 , Bernhard J Hering 6 , Stephen D Miller 7 , Xunrong Luo 2, 5
Affiliation
|
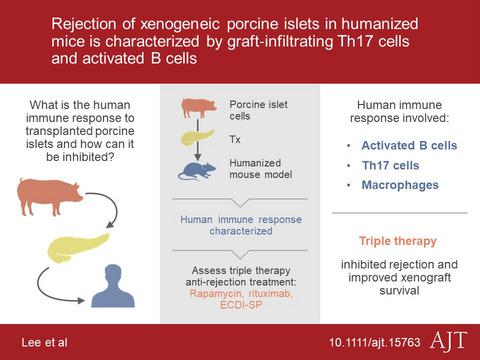
Xenogeneic porcine islet transplantation is a promising potential therapy for type 1 diabetes (T1D). Understanding human immune responses against porcine islets is crucial for the design of optimal immunomodulatory regimens for effective control of xenogeneic rejection of porcine islets in humans. Humanized mice are a valuable tool for studying human immune responses and therefore present an attractive alternative to human subject research. Here, by using a pig-to-humanized mouse model of xenogeneic islet transplantation, we described the human immune response to transplanted porcine islets, a process characterized by dense islet xenograft infiltration of human CD45+ cells comprising activated human B cells, CD4+ CD44+ IL-17+ Th17 cells, and CD68+ macrophages. In addition, we tested an experimental immunomodulatory regimen in promoting long-term islet xenograft survival, a triple therapy consisting of donor splenocytes treated with ethylcarbodiimide (ECDI-SP), and peri-transplant rituximab and rapamycin. We observed that the triple therapy effectively inhibited graft infiltration of T and B cells as well as macrophages, promoted transitional B cells both in the periphery and in the islet xenografts, and provided a superior islet xenograft protection. Our study therefore indicates an advantage of donor ECDI-SP treatment in controlling human immune cells in promoting long-term islet xenograft survival.
中文翻译:

人源化小鼠异种猪胰岛排斥的特征是移植物浸润 Th17 细胞和活化的 B 细胞。
异种猪胰岛移植是 1 型糖尿病 (T1D) 的一种很有前途的潜在疗法。了解人类对猪胰岛的免疫反应对于设计最佳免疫调节方案以有效控制人类猪胰岛异种排斥至关重要。人源化小鼠是研究人类免疫反应的宝贵工具,因此是人类课题研究的一个有吸引力的替代方案。在这里,通过使用异种胰岛移植的猪-人源化小鼠模型,我们描述了人类对移植的猪胰岛的免疫反应,这一过程的特征是人类 CD45+ 细胞的致密胰岛异种移植浸润,包括活化的人类 B 细胞、CD4+ CD44+ IL- 17+ Th17 细胞和 CD68+ 巨噬细胞。此外,我们测试了促进胰岛异种移植物长期存活的实验性免疫调节方案,这是一种三联疗法,包括用乙基碳二亚胺 (ECDI-SP) 处理的供体脾细胞,以及移植前后的利妥昔单抗和雷帕霉素。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。
更新日期:2019-12-28
中文翻译:

人源化小鼠异种猪胰岛排斥的特征是移植物浸润 Th17 细胞和活化的 B 细胞。
异种猪胰岛移植是 1 型糖尿病 (T1D) 的一种很有前途的潜在疗法。了解人类对猪胰岛的免疫反应对于设计最佳免疫调节方案以有效控制人类猪胰岛异种排斥至关重要。人源化小鼠是研究人类免疫反应的宝贵工具,因此是人类课题研究的一个有吸引力的替代方案。在这里,通过使用异种胰岛移植的猪-人源化小鼠模型,我们描述了人类对移植的猪胰岛的免疫反应,这一过程的特征是人类 CD45+ 细胞的致密胰岛异种移植浸润,包括活化的人类 B 细胞、CD4+ CD44+ IL- 17+ Th17 细胞和 CD68+ 巨噬细胞。此外,我们测试了促进胰岛异种移植物长期存活的实验性免疫调节方案,这是一种三联疗法,包括用乙基碳二亚胺 (ECDI-SP) 处理的供体脾细胞,以及移植前后的利妥昔单抗和雷帕霉素。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。我们观察到三联疗法有效地抑制了 T 细胞和 B 细胞以及巨噬细胞的移植物浸润,促进了外周和胰岛异种移植物中的移行 B 细胞,并提供了更好的胰岛异种移植物保护。因此,我们的研究表明,供体 ECDI-SP 治疗在控制人类免疫细胞以促进胰岛异种移植物长期存活方面具有优势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号