当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Observation of the Protonation States in the Mutant Green Fluorescent Protein.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpclett.9b03252 Chie Shibazaki 1 , Rumi Shimizu 1 , Yuji Kagotani 1 , Andreas Ostermann 2 , Tobias E Schrader 3 , Motoyasu Adachi 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpclett.9b03252 Chie Shibazaki 1 , Rumi Shimizu 1 , Yuji Kagotani 1 , Andreas Ostermann 2 , Tobias E Schrader 3 , Motoyasu Adachi 1
Affiliation
|
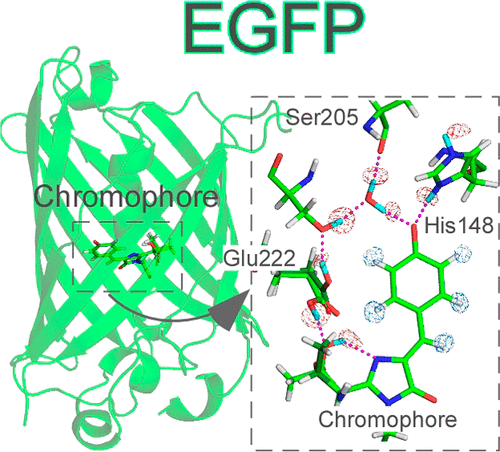
Neutron crystallography has been used to elucidate the protonation states for the enhanced green fluorescent protein, which has revolutionized imaging technologies. The structure has a deprotonated hydroxyl group in the fluorescent chromophore. Also, the protonation states of His148 and Thr203, as well as the orientation of a critical water molecule in direct contact with the chromophore, could be determined. The results demonstrate that the deprotonated hydroxyl group in the chromophore and the nitrogen atom ND1 in His148 are charged negatively and positively, respectively, forming an ion pair. The position of the two deuterium atoms in the critical water molecule appears to be displaced slightly toward the acceptor oxygen atoms according to their omit maps. This displacement implies the formation of an intriguing electrostatic potential realized inside of the protein. Our findings provide new insights into future protein design strategies along with developments in quantum chemical calculations.
中文翻译:

突变绿色荧光蛋白中质子化状态的直接观察。
中子晶体学已被用于阐明增强的绿色荧光蛋白的质子化状态,这已彻底改变了成像技术。该结构在荧光发色团中具有去质子化的羟基。而且,可以确定His148和Thr203的质子化状态,以及与生色团直接接触的关键水分子的取向。结果表明,发色团中去质子化的羟基和His148中的氮原子ND1分别带负电和正电,形成一个离子对。根据它们的省略图,临界水分子中两个氘原子的位置似乎向着受体氧原子略微偏移。这种位移暗示了在蛋白质内部实现的一种吸引人的静电势的形成。我们的发现为未来的蛋白质设计策略以及量子化学计算的发展提供了新的见识。
更新日期:2020-01-07
中文翻译:

突变绿色荧光蛋白中质子化状态的直接观察。
中子晶体学已被用于阐明增强的绿色荧光蛋白的质子化状态,这已彻底改变了成像技术。该结构在荧光发色团中具有去质子化的羟基。而且,可以确定His148和Thr203的质子化状态,以及与生色团直接接触的关键水分子的取向。结果表明,发色团中去质子化的羟基和His148中的氮原子ND1分别带负电和正电,形成一个离子对。根据它们的省略图,临界水分子中两个氘原子的位置似乎向着受体氧原子略微偏移。这种位移暗示了在蛋白质内部实现的一种吸引人的静电势的形成。我们的发现为未来的蛋白质设计策略以及量子化学计算的发展提供了新的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号