当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combination of Capillary Zone Electrophoresis-Mass Spectrometry, Ion Mobility-Mass Spectrometry, and Theoretical Calculations for Cysteine Connectivity Identification in Peptides Bearing Two Intramolecular Disulfide Bonds.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.analchem.9b03206 Cédric Delvaux 1 , Philippe Massonnet 1, 2 , Christopher Kune 1 , Jean R N Haler 1, 3 , Gregory Upert 4 , Gilles Mourier 4 , Nicolas Gilles 4 , Loïc Quinton 1 , Edwin De Pauw 1 , Johann Far 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.analchem.9b03206 Cédric Delvaux 1 , Philippe Massonnet 1, 2 , Christopher Kune 1 , Jean R N Haler 1, 3 , Gregory Upert 4 , Gilles Mourier 4 , Nicolas Gilles 4 , Loïc Quinton 1 , Edwin De Pauw 1 , Johann Far 1
Affiliation
|
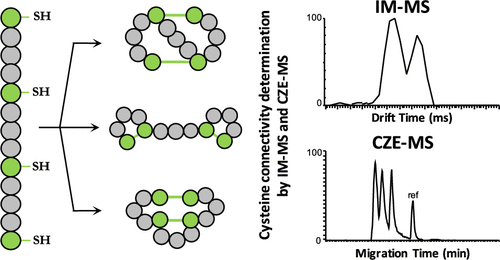
Disulfide bonds between cysteine residues are commonly involved in the stability of numerous peptides and proteins and are crucial for providing biological activities. In such peptides, the appropriate cysteine connectivity ensures the proper conformation allowing an efficient binding to their molecular targets. Disulfide bond connectivity characterization is still challenging and is a critical issue in the analysis of structured peptides/proteins targeting pharmaceutical or pharmacological utilizations. This study describes the development of new and fast gas-phase and in-solution electrophoretic methods coupled to mass spectrometry to characterize the cysteine connectivity of disulfide bonds. For this purpose, disulfide isomers of three peptides bearing two intramolecular disulfide bonds but different cysteine connectivity have been investigated. Capillary zone electrophoresis and ion mobility both coupled to mass spectrometry were used to perform the separation in both aqueous and gas phases, respectively. The separation efficiency of each technique has been critically evaluated and compared. Finally, theoretical calculations were performed to support and explain the experimental data based on the predicted physicochemical properties of the different peptides.
中文翻译:

毛细管区带电泳质谱,离子淌度质谱和理论计算相结合,用于鉴定带有两个分子内二硫键的肽的半胱氨酸连接性。
半胱氨酸残基之间的二硫键通常参与许多肽和蛋白质的稳定性,并且对于提供生物学活性至关重要。在此类肽中,适当的半胱氨酸连接性可确保适当的构象,从而使其与其分子靶标有效结合。二硫键连接性表征仍然具有挑战性,并且在针对药物或药理学用途的结构化肽/蛋白质分析中是一个关键问题。这项研究描述了新型快速气相和溶液中电泳方法与质谱联用的方法,以表征二硫键的半胱氨酸连接性。为此,已经研究了带有两个分子内二硫键但具有不同半胱氨酸连接性的三个肽的二硫键异构体。毛细管区带电泳和离子迁移率均与质谱联用,分别用于在水相和气相中进行分离。严格评估和比较了每种技术的分离效率。最后,基于预测的不同肽的理化性质,进行了理论计算以支持和解释实验数据。
更新日期:2020-01-13
中文翻译:

毛细管区带电泳质谱,离子淌度质谱和理论计算相结合,用于鉴定带有两个分子内二硫键的肽的半胱氨酸连接性。
半胱氨酸残基之间的二硫键通常参与许多肽和蛋白质的稳定性,并且对于提供生物学活性至关重要。在此类肽中,适当的半胱氨酸连接性可确保适当的构象,从而使其与其分子靶标有效结合。二硫键连接性表征仍然具有挑战性,并且在针对药物或药理学用途的结构化肽/蛋白质分析中是一个关键问题。这项研究描述了新型快速气相和溶液中电泳方法与质谱联用的方法,以表征二硫键的半胱氨酸连接性。为此,已经研究了带有两个分子内二硫键但具有不同半胱氨酸连接性的三个肽的二硫键异构体。毛细管区带电泳和离子迁移率均与质谱联用,分别用于在水相和气相中进行分离。严格评估和比较了每种技术的分离效率。最后,基于预测的不同肽的理化性质,进行了理论计算以支持和解释实验数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号