当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergetic Effect of Ru and NiO in the Electrocatalytic Decomposition of Li2CO3 to Enhance the Performance of a Li-CO2/O2 Battery
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acscatal.9b04138 Peng-Fang Zhang 1 , Jun-Yu Zhang 2 , Tian Sheng 3 , Yan-Qiu Lu 1 , Zu-Wei Yin 1 , Yu-Yang Li 2 , Xin-Xing Peng 2 , Yao Zhou 1 , Jun-Tao Li 1 , Yi-Jin Wu 1 , Jin-Xia Lin 2 , Bin-Bin Xu 2 , Xi-Ming Qu 2 , Ling Huang 2 , Shi-Gang Sun 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acscatal.9b04138 Peng-Fang Zhang 1 , Jun-Yu Zhang 2 , Tian Sheng 3 , Yan-Qiu Lu 1 , Zu-Wei Yin 1 , Yu-Yang Li 2 , Xin-Xing Peng 2 , Yao Zhou 1 , Jun-Tao Li 1 , Yi-Jin Wu 1 , Jin-Xia Lin 2 , Bin-Bin Xu 2 , Xi-Ming Qu 2 , Ling Huang 2 , Shi-Gang Sun 1, 2
Affiliation
|
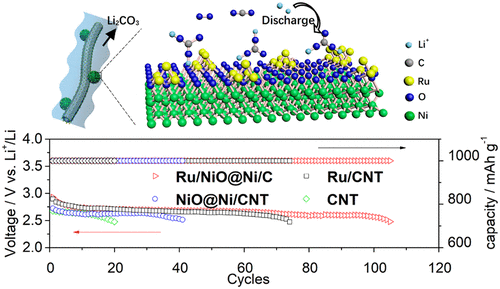
Li2CO3 is the cathodic discharge product of a Li-CO2/O2 battery and is difficult to electrochemically decompose. The accumulation of Li2CO3 leads to battery degradation and results in a short lifespan. Herein, a carbon nanotube supported Ru/[email protected] catalyst (Ru/[email protected]/CNT) is synthesized with Ru nanoparticles (∼2.5 nm) anchored on the surface of core–shell structure [email protected] nanoparticles (∼17 nm). We found strong interfacial interactions between Ru nanoparticles and NiO. XRD and XPS analysis revealed that the presence of Ru could protect the Ni species from being deeply oxidized while the NiO species could modify the local electronic structure of Ru, inducing a higher oxidation state. When such a Ru/[email protected]/CNT catalyst is used as a cathode in Li-CO2/O2 (v:v = 4:1) batteries, a long cycling life of 105 cycles at a cutoff capacity of 1000 mAh g–1 with an overpotential as low as 1.01 V was achieved, which is significantly better than 75 and 44 cycles with Ru/CNT and [email protected]/CNT catalysts, respectively, and confirms the strong synergetic effect between the Ru and NiO species in the electrocatalytic decomposition of Li2CO3. Density functional theory (DFT) calculations of the electrochemical decomposition of Li2CO3 with the assistance of RuO2 indicates that the formation of O2 is the rate-determining step. In addition, the formation and decomposition process of Li2CO3 was illuminated at a molecular level by in situ FTIR spectroscopy with Ru/[email protected]/CNT catalysts.
中文翻译:

Ru和NiO在Li 2 CO 3电催化分解中的协同作用,以增强Li-CO 2 / O 2电池的性能
Li 2 CO 3是Li-CO 2 / O 2电池的阴极放电产物,并且难以电化学分解。Li 2 CO 3的积累导致电池退化并导致使用寿命短。在此,碳纳米管负载的Ru / [受电子邮件保护的]催化剂(Ru / [受电子邮件保护的] / CNT)是通过将Ru纳米颗粒(约2.5 nm)固定在核-壳结构[受电子邮件保护的]纳米颗粒(约17)的表面上而合成的纳米)。我们发现Ru纳米颗粒与NiO之间存在很强的界面相互作用。XRD和XPS分析表明,Ru的存在可以防止Ni物种被深度氧化,而NiO可以改变Ru的局部电子结构,从而诱导较高的氧化态。当将这种Ru / [受电子邮件保护] / CNT催化剂用作Li-CO 2 / O 2(v:v = 4:1)电池的阴极时,在1000 mAh的截止容量下具有105个循环的长循环寿命g –1可以实现低至1.01 V的超电势,这明显优于分别使用Ru / CNT和[电子邮件保护] / CNT催化剂的75和44个循环,并证实了Ru和NiO物种在电催化中有很强的协同作用Li 2 CO 3的分解。借助RuO 2对Li 2 CO 3进行电化学分解的密度泛函理论(DFT)计算表明,O 2的形成是决定速率的步骤。此外,Li 2 CO 3的形成与分解过程 用Ru / [受电子邮件保护] / CNT催化剂通过原位FTIR光谱在分子水平上进行照射。
更新日期:2020-01-10
中文翻译:

Ru和NiO在Li 2 CO 3电催化分解中的协同作用,以增强Li-CO 2 / O 2电池的性能
Li 2 CO 3是Li-CO 2 / O 2电池的阴极放电产物,并且难以电化学分解。Li 2 CO 3的积累导致电池退化并导致使用寿命短。在此,碳纳米管负载的Ru / [受电子邮件保护的]催化剂(Ru / [受电子邮件保护的] / CNT)是通过将Ru纳米颗粒(约2.5 nm)固定在核-壳结构[受电子邮件保护的]纳米颗粒(约17)的表面上而合成的纳米)。我们发现Ru纳米颗粒与NiO之间存在很强的界面相互作用。XRD和XPS分析表明,Ru的存在可以防止Ni物种被深度氧化,而NiO可以改变Ru的局部电子结构,从而诱导较高的氧化态。当将这种Ru / [受电子邮件保护] / CNT催化剂用作Li-CO 2 / O 2(v:v = 4:1)电池的阴极时,在1000 mAh的截止容量下具有105个循环的长循环寿命g –1可以实现低至1.01 V的超电势,这明显优于分别使用Ru / CNT和[电子邮件保护] / CNT催化剂的75和44个循环,并证实了Ru和NiO物种在电催化中有很强的协同作用Li 2 CO 3的分解。借助RuO 2对Li 2 CO 3进行电化学分解的密度泛函理论(DFT)计算表明,O 2的形成是决定速率的步骤。此外,Li 2 CO 3的形成与分解过程 用Ru / [受电子邮件保护] / CNT催化剂通过原位FTIR光谱在分子水平上进行照射。











































 京公网安备 11010802027423号
京公网安备 11010802027423号