当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Origin of Selective Reduction of CO2 to CO on Single-Atom Nickel Catalyst.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jpcb.9b09730 Shi He 1 , Dong Ji 1 , Junwei Zhang 2 , Peter Novello 1 , Xueqian Li 1 , Qiang Zhang 2 , Xixiang Zhang 2 , Jie Liu 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jpcb.9b09730 Shi He 1 , Dong Ji 1 , Junwei Zhang 2 , Peter Novello 1 , Xueqian Li 1 , Qiang Zhang 2 , Xixiang Zhang 2 , Jie Liu 1
Affiliation
|
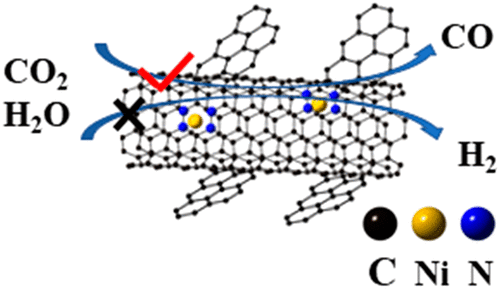
Electrochemical reduction of CO2 to CO offers a promising strategy for regulating the global carbon cycle and providing feedstock for the chemical industry. Understanding the origin that determines the faradaic efficiency (FE) of reduction of CO2 to CO is critical for developing a highly efficient electrocatalyst. Here, by constructing a single-atom Ni catalyst on nitrogen-doped winged carbon nanofiber (NiSA-NWC), we find that the single-atom Ni catalyst possesses the maximum CO FE of over 95% at -1.6 V vs Ag/AgCl, which is about 30% higher than the standard Ni nanoparticles on the same support. The Tafel analysis reveals that the single-atom Ni catalyst has a preferred reduction of CO2 to CO and a slower rate for the hydrogen evolution reaction. We propose that the domination of singular Ni1+ electronic states and limited hydrogen atom adsorption sites on the single-atom Ni catalyst lead to the observed high FE for CO2 reduction to CO.
中文翻译:

了解单原子镍催化剂上将CO2选择性还原为CO的起源。
将CO2电化学还原为CO提供了一种有前途的策略,可用于调节全球碳循环并为化学工业提供原料。了解决定CO2还原为CO的法拉第效率(FE)的起因对于开发高效电催化剂至关重要。在这里,通过在掺氮翼状碳纳米纤维(NiSA-NWC)上构建单原子Ni催化剂,我们发现,在-1.6 V时,单原子Ni催化剂相对于Ag / AgCl具有的最大CO FE超过95%,这比相同载体上的标准Ni纳米颗粒高约30%。Tafel分析表明,单原子Ni催化剂具有将CO2还原为CO的优先级,并且析氢反应的速率较慢。
更新日期:2020-01-10
中文翻译:

了解单原子镍催化剂上将CO2选择性还原为CO的起源。
将CO2电化学还原为CO提供了一种有前途的策略,可用于调节全球碳循环并为化学工业提供原料。了解决定CO2还原为CO的法拉第效率(FE)的起因对于开发高效电催化剂至关重要。在这里,通过在掺氮翼状碳纳米纤维(NiSA-NWC)上构建单原子Ni催化剂,我们发现,在-1.6 V时,单原子Ni催化剂相对于Ag / AgCl具有的最大CO FE超过95%,这比相同载体上的标准Ni纳米颗粒高约30%。Tafel分析表明,单原子Ni催化剂具有将CO2还原为CO的优先级,并且析氢反应的速率较慢。











































 京公网安备 11010802027423号
京公网安备 11010802027423号