当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural responses of kiwifruit allergen Act d 2 to thermal and electric field stresses based on molecular dynamics simulations and experiments.
Food & Function ( IF 5.1 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9fo02427a Jin Wang 1 , Sai Kranthi Vanga , Vijaya Raghavan
Food & Function ( IF 5.1 ) Pub Date : 2020-01-22 , DOI: 10.1039/c9fo02427a Jin Wang 1 , Sai Kranthi Vanga , Vijaya Raghavan
Affiliation
|
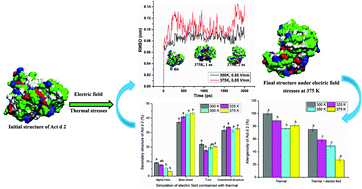
Kiwifruit is considered to be the most common plant-based food causing allergic reactions, after peanuts, soybeans, and wheat. These responses are triggered by the proteins in kiwifruit. Modifying the structures of these allergenic proteins (epitopes) through external stresses may decrease their specific binding capacity with antibodies, resulting in a decrease in kiwifruit allergenicity. To provide a visual insight into the structural changes of Act d 2, we treated it with a thermal force and an oscillating electric field using molecular dynamics (MD) simulations. The results showed that Act d 2 is a heat-stable protein, while the electric field combined with thermal stress significantly affected its secondary structure and surface properties, which could result in conformational changes. The results obtained from ELISA tests showed that the combination of the thermal force and electric field significantly reduced the Ig-E binding capacity of Act d 2 by 75.3%, accompanied with obvious losses of alpha-helix and turn structures.
中文翻译:

基于分子动力学模拟和实验,猕猴桃过敏原Act d 2对热和电场应力的结构响应。
猕猴桃被认为是引起过敏反应的最常见的植物性食品,仅次于花生,大豆和小麦。这些反应是由奇异果中的蛋白质触发的。通过外部压力修饰这些过敏原蛋白(表位)的结构可能会降低其与抗体的特异性结合能力,从而导致猕猴桃过敏原性降低。为了直观地了解Act d 2的结构变化,我们使用分子动力学(MD)模拟以热力和振荡电场对其进行了处理。结果表明,Act d 2是一种热稳定蛋白,而电场与热应力的结合显着影响其二级结构和表面性质,这可能导致构象变化。
更新日期:2020-02-26
中文翻译:

基于分子动力学模拟和实验,猕猴桃过敏原Act d 2对热和电场应力的结构响应。
猕猴桃被认为是引起过敏反应的最常见的植物性食品,仅次于花生,大豆和小麦。这些反应是由奇异果中的蛋白质触发的。通过外部压力修饰这些过敏原蛋白(表位)的结构可能会降低其与抗体的特异性结合能力,从而导致猕猴桃过敏原性降低。为了直观地了解Act d 2的结构变化,我们使用分子动力学(MD)模拟以热力和振荡电场对其进行了处理。结果表明,Act d 2是一种热稳定蛋白,而电场与热应力的结合显着影响其二级结构和表面性质,这可能导致构象变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号