Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple features within the syntaxin Sed5p mediate its Golgi localization.
Traffic ( IF 3.6 ) Pub Date : 2019-12-27 , DOI: 10.1111/tra.12720 Guanbin Gao 1 , David K Banfield 1
Traffic ( IF 3.6 ) Pub Date : 2019-12-27 , DOI: 10.1111/tra.12720 Guanbin Gao 1 , David K Banfield 1
Affiliation
|
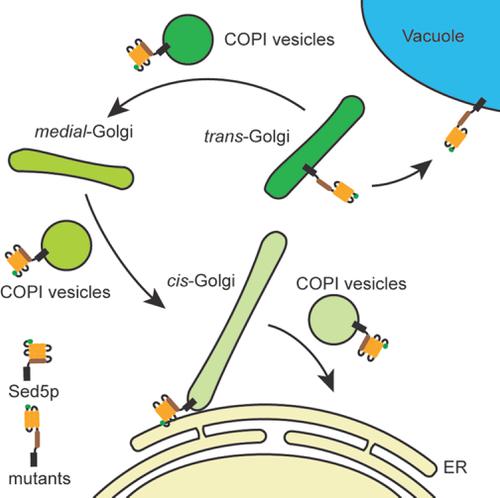
Protein retention and the transport of proteins and lipids into and out of the Golgi is intimately linked to the biogenesis and homeostasis of this sorting hub of eukaryotic cells. Of particular importance are membrane proteins that mediate membrane fusion events with and within the Golgi-the Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs). In the Golgi of budding yeast cells, the syntaxin SNARE Sed5p oversees membrane fusion events. Determining how Sed5p is localized to and trafficked within the Golgi is critical to informing our understanding of the mechanism(s) of biogenesis and homeostasis of this organelle. Here we establish that the steady-state localization of Sed5p to the Golgi appears to be primarily conformation-based relying on intra-molecular associations between the Habc domain and SNARE-motif while its tribasic COPI-coatomer binding motif plays a role in intra-Golgi retention.
中文翻译:

Sed5p语法中的多个功能可调节其高尔基语本地化。
蛋白质保留以及蛋白质和脂质进出高尔基体的运输与真核细胞分选中心的生物发生和体内平衡密切相关。特别重要的是与高尔基体-可溶性N-乙基马来酰亚胺敏感因子附着蛋白受体(SNARE)一起并在其中发生膜融合事件的膜蛋白。在发芽酵母细胞的高尔基体中,语法SNARE Sed5p监督膜融合事件。确定Sed5p如何定位于高尔基体并在其中迁移是至关重要的,这有助于我们了解该细胞器的生物发生和体内稳态机制。
更新日期:2020-02-18
中文翻译:

Sed5p语法中的多个功能可调节其高尔基语本地化。
蛋白质保留以及蛋白质和脂质进出高尔基体的运输与真核细胞分选中心的生物发生和体内平衡密切相关。特别重要的是与高尔基体-可溶性N-乙基马来酰亚胺敏感因子附着蛋白受体(SNARE)一起并在其中发生膜融合事件的膜蛋白。在发芽酵母细胞的高尔基体中,语法SNARE Sed5p监督膜融合事件。确定Sed5p如何定位于高尔基体并在其中迁移是至关重要的,这有助于我们了解该细胞器的生物发生和体内稳态机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号