当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron Paramagnetic Resonance and Magnetic Circular Dichroism Spectra of the Nitrogenase M Cluster Precursor Suggest Sulfur Migration upon Oxidation: A Proposal for Substrate and Inhibitor Binding.
ChemBioChem ( IF 2.6 ) Pub Date : 2019-12-27 , DOI: 10.1002/cbic.201900681 Kresimir Rupnik 1 , Kazuki Tanifuji 2 , Lee Rettberg 2 , Markus W Ribbe 2, 3 , Yilin Hu 2 , Brian J Hales 1
ChemBioChem ( IF 2.6 ) Pub Date : 2019-12-27 , DOI: 10.1002/cbic.201900681 Kresimir Rupnik 1 , Kazuki Tanifuji 2 , Lee Rettberg 2 , Markus W Ribbe 2, 3 , Yilin Hu 2 , Brian J Hales 1
Affiliation
|
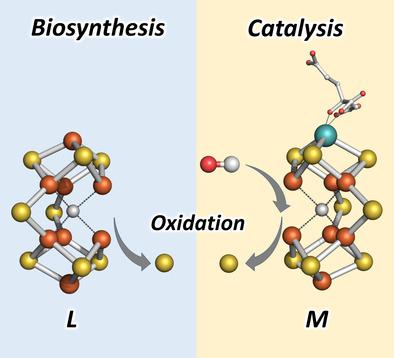
Belt out : Combined EPR and MCD analyses show the migration of a belt sulfur away from the nitrogenase cofactor precursor, L , upon oxidation. A similar migration is proposed for the nitrogenase cofactor, M , upon turnover. Such an oxidation‐induced labilization of the cofactor belt region could be instrumental in nitrogenase cofactor biosynthesis and substrate/inhibitor binding.

中文翻译:

固氮酶 M 簇前体的电子顺磁共振和磁圆二色性光谱表明氧化时硫迁移:底物和抑制剂结合的建议。
Belt out :结合 EPR 和 MCD 分析显示,在氧化时,带硫从固氮酶辅因子前体L迁移走。固氮酶辅助因子M在周转时也会发生类似的迁移。这种氧化诱导的辅因子带区域的不稳定可能有助于固氮酶辅因子生物合成和底物/抑制剂结合。

更新日期:2019-12-27

中文翻译:

固氮酶 M 簇前体的电子顺磁共振和磁圆二色性光谱表明氧化时硫迁移:底物和抑制剂结合的建议。
Belt out :结合 EPR 和 MCD 分析显示,在氧化时,带硫从固氮酶辅因子前体L迁移走。固氮酶辅助因子M在周转时也会发生类似的迁移。这种氧化诱导的辅因子带区域的不稳定可能有助于固氮酶辅因子生物合成和底物/抑制剂结合。












































 京公网安备 11010802027423号
京公网安备 11010802027423号