当前位置:
X-MOL 学术
›
Bioelectrochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Luminal addition of non-permeant Eu3+ interferes with luminal Ca2+ regulation of the cardiac ryanodine receptor.
Bioelectrochemistry ( IF 5 ) Pub Date : 2019-12-28 , DOI: 10.1016/j.bioelechem.2019.107449 Jana Gaburjakova 1 , Janos Almassy 2 , Marta Gaburjakova 1
Bioelectrochemistry ( IF 5 ) Pub Date : 2019-12-28 , DOI: 10.1016/j.bioelechem.2019.107449 Jana Gaburjakova 1 , Janos Almassy 2 , Marta Gaburjakova 1
Affiliation
|
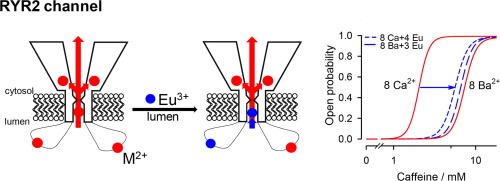
Dysregulation of the cardiac ryanodine receptor (RYR2) by luminal Ca2+ has been implicated in a life-threatening, stress-induced arrhythmogenic disease. The mechanism of luminal Ca2+-mediated RYR2 regulation is under debate, and it has been attributed to Ca2+ binding on the cytosolic face (the Ca2+ feedthrough mechanism) and/or the luminal face of the RYR2 channel (the true luminal mechanism). The molecular nature and location of the luminal Ca2+ site is unclear. At the single-channel level, we directly probed the RYR2 luminal face by Eu3+, considering the non-permeant nature of trivalent cations and their high binding affinities for Ca2+ sites. Without affecting essential determinants of the Ca2+ feedthrough mechanism, we found that luminal Eu3+ competitively antagonized the activation effect of luminal Ca2+ on RYR2 responsiveness to cytosolic caffeine, and no appreciable effect was observed for luminal Ba2+ (mimicking the absence of luminal Ca2+). Importantly, luminal Eu3+ caused no changes in RYR2 gating. Our results indicate that two distinct Ca2+ sites (available for luminal Ca2+ even when the channel is closed) are likely involved in the true luminal mechanism. One site facing the lumen regulates channel responsiveness to caffeine, while the other site, presumably positioned in the channel pore, governs the gating behavior.
中文翻译:

夜光添加非渗透性Eu3 +会干扰心脏ryanodine受体的腔内Ca2 +调节。
腔内Ca2 +对心脏ryanodine受体(RYR2)的调节失调与威胁生命的应激诱发的心律失常疾病有关。腔内Ca2 +介导的RYR2调控的机制尚有争议,并且已归因于Ca2 +结合在RYR2通道的胞质面上(Ca2 +馈通机制)和/或腔内表面(真正的腔内机制)。腔内Ca2 +位点的分子性质和位置尚不清楚。在单通道水平上,考虑到三价阳离子的非渗透性及其对Ca2 +位点的高结合亲和力,我们通过Eu3 +直接探测了RYR2腔表面。在不影响Ca2 +馈通机制的基本决定因素的情况下,我们发现,腔Eu3 +竞争性拮抗腔Ca2 +对RYR2对胞质咖啡因的反应的激活作用,并且腔Ba2 +没有观察到明显的作用(模仿了腔Ca2 +的缺失)。重要的是,腔内Eu3 +不会引起RYR2门控的变化。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。
更新日期:2019-12-29
中文翻译:

夜光添加非渗透性Eu3 +会干扰心脏ryanodine受体的腔内Ca2 +调节。
腔内Ca2 +对心脏ryanodine受体(RYR2)的调节失调与威胁生命的应激诱发的心律失常疾病有关。腔内Ca2 +介导的RYR2调控的机制尚有争议,并且已归因于Ca2 +结合在RYR2通道的胞质面上(Ca2 +馈通机制)和/或腔内表面(真正的腔内机制)。腔内Ca2 +位点的分子性质和位置尚不清楚。在单通道水平上,考虑到三价阳离子的非渗透性及其对Ca2 +位点的高结合亲和力,我们通过Eu3 +直接探测了RYR2腔表面。在不影响Ca2 +馈通机制的基本决定因素的情况下,我们发现,腔Eu3 +竞争性拮抗腔Ca2 +对RYR2对胞质咖啡因的反应的激活作用,并且腔Ba2 +没有观察到明显的作用(模仿了腔Ca2 +的缺失)。重要的是,腔内Eu3 +不会引起RYR2门控的变化。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。我们的结果表明,两个不同的Ca2 +位点(即使通道关闭也可用于腔Ca2 +)可能参与了真正的腔机理。面向管腔的一个部位调节通道对咖啡因的响应,而另一部位(可能位于通道孔中)控制选通行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号