当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid-Phase Plasma-Assisted in Situ Synthesis of Amino-Rich Nanocarbon for Transition Metal Ion Adsorption
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acsanm.9b01915 Mongkol Tipplook 1 , Phuwadej Pornaroontham 1, 2 , Anyarat Watthanaphanit 3 , Nagahiro Saito 1, 2, 4, 5, 6
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-01-09 , DOI: 10.1021/acsanm.9b01915 Mongkol Tipplook 1 , Phuwadej Pornaroontham 1, 2 , Anyarat Watthanaphanit 3 , Nagahiro Saito 1, 2, 4, 5, 6
Affiliation
|
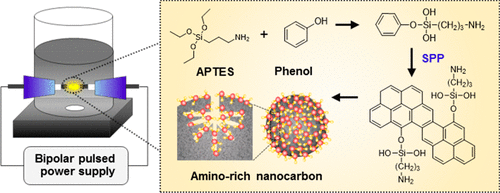
Amino-modified nanocarbon (NH2-C) has been widely used as an adsorbent for transition metal ion adsorption due to its high specific surface area, high electrical charge, and the ability to form a coordinate linkage to a transition metal ion. In this work, NH2-C was successfully synthesized from a mixture of phenol and (3-aminopropyl)triethoxysilane (APTES) through solution plasma processing, which performs both carbonization and amination simultaneously. This synthesis method eliminates the need to functionalize carbon with amino groups, as is required in the conventional process. Our NH2-C shows a better dispersion and a higher number of amino groups on both the external surface and inner pores, which enhances the adsorption capacity. The maximum capacities for Cu2+, Zn2+, and Cd2+ adsorption were 144.9, 115.4, and 102.0 mg g–1, respectively. These values were higher than those of five typical NH2-Cs synthesized by a conventional process. Based on the adsorption mechanism derived from adsorption kinetic, isotherm, and thermodynamic studies, the transition metal ions were chemisorbed to the surface in a monolayer endothermically and spontaneously. Moreover, it was found that NH2-C was suitable for use in ten consecutive adsorption–desorption cycles without significant loss of adsorption capacity.
中文翻译:

液相等离子体辅助原位合成富含氨基的纳米碳,用于过渡金属离子的吸附
氨基改性的纳米碳(NH 2 -C)由于其高比表面积,高电荷以及与过渡金属离子形成配位键的能力而被广泛用作过渡金属离子吸附的吸附剂。在这项工作中,通过溶液等离子体处理成功地从苯酚和(3-氨基丙基)三乙氧基硅烷(APTES)的混合物合成了NH 2 -C,该过程同时进行碳化和胺化。这种合成方法消除了常规方法所需要的用氨基官能化碳的需要。我们的NH 2-C在外表面和内孔上均显示出较好的分散性和较高数量的氨基,从而提高了吸附能力。Cu 2 +,Zn 2+和Cd 2+的最大吸附量分别为144.9、115.4和102.0 mg g –1。这些值高于通过常规方法合成的五个典型的NH 2 -C的值。基于从吸附动力学,等温线和热力学研究得出的吸附机理,过渡金属离子以吸热和自发的方式化学吸附在单层表面。此外,发现NH 2-C适用于连续十次吸附-解吸循环,而不会显着降低吸附能力。
更新日期:2020-01-10
中文翻译:

液相等离子体辅助原位合成富含氨基的纳米碳,用于过渡金属离子的吸附
氨基改性的纳米碳(NH 2 -C)由于其高比表面积,高电荷以及与过渡金属离子形成配位键的能力而被广泛用作过渡金属离子吸附的吸附剂。在这项工作中,通过溶液等离子体处理成功地从苯酚和(3-氨基丙基)三乙氧基硅烷(APTES)的混合物合成了NH 2 -C,该过程同时进行碳化和胺化。这种合成方法消除了常规方法所需要的用氨基官能化碳的需要。我们的NH 2-C在外表面和内孔上均显示出较好的分散性和较高数量的氨基,从而提高了吸附能力。Cu 2 +,Zn 2+和Cd 2+的最大吸附量分别为144.9、115.4和102.0 mg g –1。这些值高于通过常规方法合成的五个典型的NH 2 -C的值。基于从吸附动力学,等温线和热力学研究得出的吸附机理,过渡金属离子以吸热和自发的方式化学吸附在单层表面。此外,发现NH 2-C适用于连续十次吸附-解吸循环,而不会显着降低吸附能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号