当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dimerization of LOV domains of Rhodobacter sphaeroides (RsLOV) studied with FRET and stopped-flow experiments.
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-10 , DOI: 10.1039/c9pp00424f Kathrin Magerl 1 , Bernhard Dick
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-10 , DOI: 10.1039/c9pp00424f Kathrin Magerl 1 , Bernhard Dick
Affiliation
|
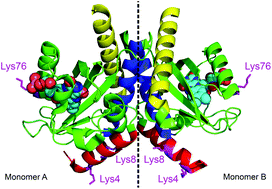
The bacterium Rhodobacter sphaeroides has a short LOV (light-oxygen-voltage) domain, which is not connected to an effector domain but has an α-helix extension at the N-terminus as well as a helix-turn-helix (HTH) motiv at the C-terminus. These extensions offer possibilities for interactions with effector enzymes or DNA. Whereas many LOV domains show a tendency to form dimers in the light state, RsLOV is unique in that it is a dimer in the dark state but dissociates into monomers after blue-light excitation. We studied the kinetics of this dimerization process by a combination of FRET spectroscopy and stopped-flow experiments with a time resolution of ≈10 ms. Although excitation of the flavin chromophore in dye-labeled LOV domains leads to considerable FRET from flavin to the dye, the typical adduct formation between flavin and a nearby cysteine still occurs with considerable yield. We obtain a rate constant for LOV-LOV dimerization in the range (0.8-1.8) × 105 M-1 s-1, and an equilibrium constant of the dark-state dimer in the range (3.0-7.0) × 10-6 M. Dissociation of the dimers in the light state and reforming of dimers after return to the dark state was monitored using an anti-FRET effect caused by excitonic interaction between dye labels on different monomers. Reforming of the dark state dimers is slower than recovery of the flavin-cysteinyl adduct, indicating that light-induced conformational changes in the LOV domain persist for much longer time than the adduct lifetime.
中文翻译:

用FRET和停流实验研究球形球形红细菌(RsLOV)的LOV域的二聚化。
球形红球细菌具有短的LOV(光-氧-电压)结构域,该结构域未连接到效应子结构域,但在N端具有α-螺旋延伸,并且具有螺旋-转-螺旋(HTH)动机在C端。这些扩展为与效应酶或DNA相互作用提供了可能性。尽管许多LOV域显示出在明亮状态下形成二聚体的趋势,但RsLOV的独特之处在于,它在黑暗状态下为二聚体,但在蓝光激发后会解离为单体。我们通过FRET光谱学和停止流实验相结合,以约10 ms的时间分辨率研究了该二聚过程的动力学。尽管在染料标记的LOV域中激发黄素发色团会导致从黄素到染料的大量FRET,黄素与附近半胱氨酸之间的典型加合物形成仍会以相当高的收率发生。我们获得了LOV-LOV二聚化的速率常数,范围为(0.8-1.8)×105 M-1 s-1,暗态二聚体的平衡常数为(3.0-7.0)×10-6 M使用由不同单体上的染料标记之间的激子相互作用引起的抗FRET效应来监测亮态下的二聚体的解离和返回暗态后的二聚体的重整。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。且暗态二聚体的平衡常数在(3.0-7.0)×10-6 M范围内。使用抗FRET效应监测亮态二聚体的解离和返回暗态后二聚体的重整。由不同单体上的染料标记之间的激子相互作用引起的。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。且暗态二聚体的平衡常数在(3.0-7.0)×10-6 M范围内。使用抗FRET效应监测亮态二聚体的解离和返回暗态后二聚体的重整。由不同单体上的染料标记之间的激子相互作用引起的。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。
更新日期:2020-02-19
中文翻译:

用FRET和停流实验研究球形球形红细菌(RsLOV)的LOV域的二聚化。
球形红球细菌具有短的LOV(光-氧-电压)结构域,该结构域未连接到效应子结构域,但在N端具有α-螺旋延伸,并且具有螺旋-转-螺旋(HTH)动机在C端。这些扩展为与效应酶或DNA相互作用提供了可能性。尽管许多LOV域显示出在明亮状态下形成二聚体的趋势,但RsLOV的独特之处在于,它在黑暗状态下为二聚体,但在蓝光激发后会解离为单体。我们通过FRET光谱学和停止流实验相结合,以约10 ms的时间分辨率研究了该二聚过程的动力学。尽管在染料标记的LOV域中激发黄素发色团会导致从黄素到染料的大量FRET,黄素与附近半胱氨酸之间的典型加合物形成仍会以相当高的收率发生。我们获得了LOV-LOV二聚化的速率常数,范围为(0.8-1.8)×105 M-1 s-1,暗态二聚体的平衡常数为(3.0-7.0)×10-6 M使用由不同单体上的染料标记之间的激子相互作用引起的抗FRET效应来监测亮态下的二聚体的解离和返回暗态后的二聚体的重整。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。且暗态二聚体的平衡常数在(3.0-7.0)×10-6 M范围内。使用抗FRET效应监测亮态二聚体的解离和返回暗态后二聚体的重整。由不同单体上的染料标记之间的激子相互作用引起的。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。且暗态二聚体的平衡常数在(3.0-7.0)×10-6 M范围内。使用抗FRET效应监测亮态二聚体的解离和返回暗态后二聚体的重整。由不同单体上的染料标记之间的激子相互作用引起的。暗态二聚体的重整比黄素-半胱氨酸加合物的恢复慢,表明LOV域中光诱导的构象变化比加合物寿命持续更长的时间。









































 京公网安备 11010802027423号
京公网安备 11010802027423号