Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic modeling of spontaneous penetration of carbon nanocones into membrane vesicles.
Nanoscale ( IF 5.8 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9nr09098c Shuo Wang 1 , Xuejin Li 2 , Xiaobo Gong 1 , Haojun Liang 3
Nanoscale ( IF 5.8 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9nr09098c Shuo Wang 1 , Xuejin Li 2 , Xiaobo Gong 1 , Haojun Liang 3
Affiliation
|
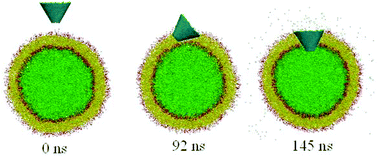
Carbon nanocones (CNCs) are promising drug delivery systems that can be functionalized with a variety of biomolecules (such as proteins, peptides, or antibodies), which allow for site-specific, targeted payload delivery to particular cells and organs. However, considerable uncertainty exists with respect to the toxicity of CNCs on their conical shape, and the underlying mechanism that leads to the penetration of CNCs (especially the truncated ones) in and through the cell membrane is not yet well understood. Using a coarse-grained dissipative particle dynamics method, we systematically investigate the spontaneous penetration of untruncated and truncated CNCs into membrane vesicles. For untruncated CNCs, the simulation results show that both pristine and oxidized ones can spontaneously penetrate across or be attached to the vesicle surface without membrane rupture, indicating low or insignificant toxicity. However, for truncated CNCs, we find that both the apex angle and aspect ratio can influence the CNC-membrane interactions and CNC-induced toxicity: a higher apex angle (and/or a lower aspect ratio) yields a higher toxicity of truncated CNCs. Further free energy analysis reveals that the lowest free energy path during the penetration is associated with CNC's orientation and rotation. For a truncated CNC with a low aspect ratio and high apex angle, it tends to rotate itself to a preferred standing-up fashion inside the vesicle membrane, posing an enhanced toxicity of CNCs. These findings may provide useful guidelines for designing effective CNC vehicles for drug delivery.
中文翻译:

碳纳米锥自发渗入膜囊泡的机理模型。
碳纳米锥(CNC)是有前途的药物输送系统,可以用多种生物分子(例如蛋白质,肽或抗体)功能化,从而可以将位点特异性的靶向有效载荷输送到特定的细胞和器官。然而,关于氯化萘在其圆锥形上的毒性,存在相当大的不确定性,导致氯化萘(尤其是截短的氯化萘)在细胞膜内和通过细胞膜渗透的潜在机制尚未得到很好的理解。使用粗粒耗散粒子动力学方法,我们系统地研究未截断的和截断的CNCs自发渗透到膜囊泡中。对于不删节的CNC,模拟结果表明,原始的和氧化的均可自发穿透或附着在囊泡表面,而不会发生膜破裂,表明毒性低或微不足道。然而,对于截短的CNC,我们发现顶角和长宽比均会影响CNC膜相互作用和CNC诱导的毒性:顶角更大(和/或更低的长宽比)会产生截短的CNC更高的毒性。进一步的自由能分析表明,穿透过程中最低的自由能路径与CNC的方向和旋转有关。对于具有低长宽比和高顶角的截短型CNC,它倾向于将自身旋转到囊泡膜内以一种优选的直立方式旋转,从而增强了CNC的毒性。
更新日期:2020-01-09
中文翻译:

碳纳米锥自发渗入膜囊泡的机理模型。
碳纳米锥(CNC)是有前途的药物输送系统,可以用多种生物分子(例如蛋白质,肽或抗体)功能化,从而可以将位点特异性的靶向有效载荷输送到特定的细胞和器官。然而,关于氯化萘在其圆锥形上的毒性,存在相当大的不确定性,导致氯化萘(尤其是截短的氯化萘)在细胞膜内和通过细胞膜渗透的潜在机制尚未得到很好的理解。使用粗粒耗散粒子动力学方法,我们系统地研究未截断的和截断的CNCs自发渗透到膜囊泡中。对于不删节的CNC,模拟结果表明,原始的和氧化的均可自发穿透或附着在囊泡表面,而不会发生膜破裂,表明毒性低或微不足道。然而,对于截短的CNC,我们发现顶角和长宽比均会影响CNC膜相互作用和CNC诱导的毒性:顶角更大(和/或更低的长宽比)会产生截短的CNC更高的毒性。进一步的自由能分析表明,穿透过程中最低的自由能路径与CNC的方向和旋转有关。对于具有低长宽比和高顶角的截短型CNC,它倾向于将自身旋转到囊泡膜内以一种优选的直立方式旋转,从而增强了CNC的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号