Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of food intake by astrocytes in the brainstem dorsal vagal complex.
Glia ( IF 5.4 ) Pub Date : 2019-12-27 , DOI: 10.1002/glia.23774 Alastair J MacDonald 1, 2 , Fiona E Holmes 2 , Craig Beall 1 , Anthony E Pickering 2, 3 , Kate L J Ellacott 1
Glia ( IF 5.4 ) Pub Date : 2019-12-27 , DOI: 10.1002/glia.23774 Alastair J MacDonald 1, 2 , Fiona E Holmes 2 , Craig Beall 1 , Anthony E Pickering 2, 3 , Kate L J Ellacott 1
Affiliation
|
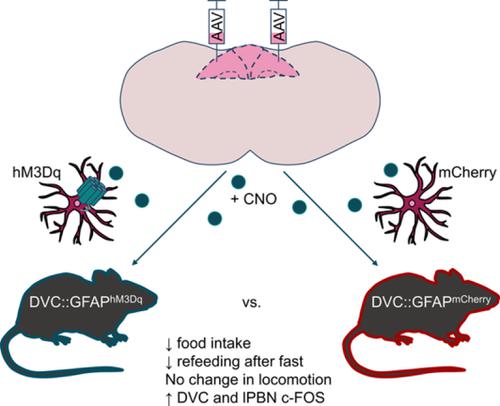
A role for glial cells in brain circuits controlling feeding has begun to be identified with hypothalamic astrocyte signaling implicated in regulating energy homeostasis. The nucleus of the solitary tract (NTS), within the brainstem dorsal vagal complex (DVC), integrates vagal afferent information from the viscera and plays a role in regulating food intake. We hypothesized that astrocytes in this nucleus respond to, and influence, food intake. Mice fed high-fat chow for 12 hr during the dark phase showed NTS astrocyte activation, reflected in an increase in the number (65%) and morphological complexity of glial-fibrillary acidic protein (GFAP)-immunoreactive cells adjacent to the area postrema (AP), compared to control chow fed mice. To measure the impact of astrocyte activation on food intake, we delivered designer receptors exclusively activated by designer drugs (DREADDs) to DVC astrocytes (encompassing NTS, AP, and dorsal motor nucleus of the vagus) using an adeno-associated viral (AAV) vector (AAV-GFAP-hM3Dq_mCherry). Chemogenetic activation with clozapine-N-oxide (0.3 mg/kg) produced in greater morphological complexity in astrocytes and reduced dark-phase feeding by 84% at 4 hr postinjection compared with vehicle treatment. hM3Dq-activation of DVC astrocytes also reduced refeeding after an overnight fast (71% lower, 4 hr postinjection) when compared to AAV-GFAP-mCherry expressing control mice. DREADD-mediated astrocyte activation did not impact locomotion. hM3Dq activation of DVC astrocytes induced c-FOS in neighboring neuronal feeding circuits (including in the parabrachial nucleus). This indicates that NTS astrocytes respond to acute nutritional excess, are involved in the integration of peripheral satiety signals, and can reduce food intake when activated.
中文翻译:

脑干背侧迷走神经复合体中星形胶质细胞对食物摄入的调节。
神经胶质细胞在控制进食的大脑回路中的作用已开始通过下丘脑星形胶质细胞信号传导参与调节能量稳态。脑干背侧迷走神经复合体 (DVC) 内的孤束核 (NTS) 整合来自内脏的迷走神经传入信息,并在调节食物摄入方面发挥作用。我们假设该细胞核中的星形胶质细胞对食物摄入有反应并影响食物摄入。在黑暗阶段喂食高脂肪食物 12 小时的小鼠显示出 NTS 星形胶质细胞活化,这反映在邻近区域的胶质纤维酸性蛋白 (GFAP) 免疫反应细胞的数量 (65%) 和形态复杂性增加。 AP),与对照饲料喂养的小鼠相比。为了测量星形胶质细胞活化对食物摄入的影响,我们使用腺相关病毒 (AAV) 载体 (AAV-GFAP-hM3Dq_mCherry) 将仅由设计药物 (DREADD) 激活的设计受体传递到 DVC 星形胶质细胞(包括 NTS、AP 和迷走神经背侧运动核)。与载体治疗相比,氯氮平-N-氧化物 (0.3 mg/kg) 的化学活化在星形胶质细胞中产生更大的形态复杂性,并在注射后 4 小时将暗相喂养减少 84%。与表达 AAV-GFAP-mCherry 的对照小鼠相比,hM3Dq 激活 DVC 星形胶质细胞也减少了隔夜禁食后的再喂养(降低 71%,注射后 4 小时)。DREADD 介导的星形胶质细胞激活不影响运动。DVC 星形胶质细胞的 hM3Dq 激活在邻近的神经元喂养回路(包括臂旁核)中诱导 c-FOS。
更新日期:2019-12-27
中文翻译:

脑干背侧迷走神经复合体中星形胶质细胞对食物摄入的调节。
神经胶质细胞在控制进食的大脑回路中的作用已开始通过下丘脑星形胶质细胞信号传导参与调节能量稳态。脑干背侧迷走神经复合体 (DVC) 内的孤束核 (NTS) 整合来自内脏的迷走神经传入信息,并在调节食物摄入方面发挥作用。我们假设该细胞核中的星形胶质细胞对食物摄入有反应并影响食物摄入。在黑暗阶段喂食高脂肪食物 12 小时的小鼠显示出 NTS 星形胶质细胞活化,这反映在邻近区域的胶质纤维酸性蛋白 (GFAP) 免疫反应细胞的数量 (65%) 和形态复杂性增加。 AP),与对照饲料喂养的小鼠相比。为了测量星形胶质细胞活化对食物摄入的影响,我们使用腺相关病毒 (AAV) 载体 (AAV-GFAP-hM3Dq_mCherry) 将仅由设计药物 (DREADD) 激活的设计受体传递到 DVC 星形胶质细胞(包括 NTS、AP 和迷走神经背侧运动核)。与载体治疗相比,氯氮平-N-氧化物 (0.3 mg/kg) 的化学活化在星形胶质细胞中产生更大的形态复杂性,并在注射后 4 小时将暗相喂养减少 84%。与表达 AAV-GFAP-mCherry 的对照小鼠相比,hM3Dq 激活 DVC 星形胶质细胞也减少了隔夜禁食后的再喂养(降低 71%,注射后 4 小时)。DREADD 介导的星形胶质细胞激活不影响运动。DVC 星形胶质细胞的 hM3Dq 激活在邻近的神经元喂养回路(包括臂旁核)中诱导 c-FOS。











































 京公网安备 11010802027423号
京公网安备 11010802027423号