当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective Hydroxylation and Alkoxylation of Silanes: One-Pot Silane Oxidation and Reduction of Aldehydes/Ketones
Organometallics ( IF 2.5 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.organomet.9b00716 Nianhua Luo 1 , Jianhua Liao 1, 2 , Lu Ouyang 1 , Huiling Wen 1 , Yuhong Zhong 1 , Jitian Liu 2 , Weiping Tang 2 , Renshi Luo 1
Organometallics ( IF 2.5 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.organomet.9b00716 Nianhua Luo 1 , Jianhua Liao 1, 2 , Lu Ouyang 1 , Huiling Wen 1 , Yuhong Zhong 1 , Jitian Liu 2 , Weiping Tang 2 , Renshi Luo 1
Affiliation
|
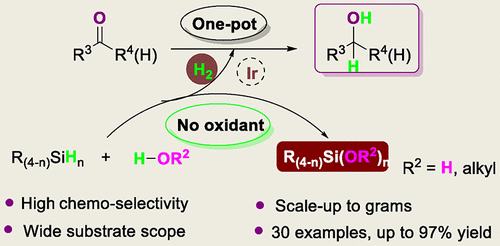
An efficient chemoselective iridium-catalyzed method for the hydroxylation and alkoxylation of organosilanes to generate hydrogen gas and silanols or silyl ethers was developed. A variety of sterically hindered silanes with alkyl, aryl, and ether groups were tolerated. Furthermore, this atom-economical catalytic protocol can be used for the synthesis of silanediols and silanetriols. A one-pot silane oxidation and chemoselective reduction of aldehydes/ketones was also realized.
中文翻译:

硅烷的高度选择性羟基化和烷氧基化:一锅硅烷氧化和醛/酮的还原
开发了一种有效的化学选择性铱催化的方法,用于有机硅烷的羟基化和烷氧基化以生成氢气和硅烷醇或甲硅烷基醚。可以耐受各种具有烷基,芳基和醚基的空间位阻硅烷。此外,这种原子经济的催化方案可用于硅烷二醇和硅烷三醇的合成。还实现了一锅硅烷氧化和醛/酮的化学选择性还原。
更新日期:2019-12-27
中文翻译:

硅烷的高度选择性羟基化和烷氧基化:一锅硅烷氧化和醛/酮的还原
开发了一种有效的化学选择性铱催化的方法,用于有机硅烷的羟基化和烷氧基化以生成氢气和硅烷醇或甲硅烷基醚。可以耐受各种具有烷基,芳基和醚基的空间位阻硅烷。此外,这种原子经济的催化方案可用于硅烷二醇和硅烷三醇的合成。还实现了一锅硅烷氧化和醛/酮的化学选择性还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号