Micron ( IF 2.5 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.micron.2019.102817 Firomsa Teshale 1 , R Karthikeyan 2 , Omprakash Sahu 3
|
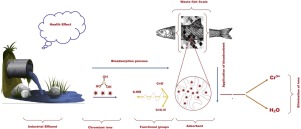
Presence of heavy metal in industrial wastewater is hazardous to the surrounding environment. Biosorption of heavy metal is an effective technology for the treatment of industrial wastewater. This research work has been carried out on removal of chromium (III) metal ions by employing waste fish scales as bioadsorbent. A batch adsorption process was carried out with different adsorbent dosage, solution pH and contact time. The results show the highest 99.7518 % chromium (III) metal ions at bioadsorbent dosage 0.8 g, pH of the solution 5 and contact time 90 min, initial concentration 150 mg/l chromium ion. The adsorption isotherms data fitted well with the Langmuir isotherm model with R2 = 0.9998, qmax = 18.3486 mg/g, and RL = 0.00007325. As well as pseudo-first and second kinetics model was also analyzed for the description of adsorption and found to be well fitted (R2 = 1) for adsorption kinetics. The surface properties activated fish scales and chromium loaded fish scale were investigated by scanning electron microscopy, X-ray spectroscopy, Fourier-transform infrared spectroscopy, and thermal analysis and agree with outcomes.
中文翻译:

鱼鳞合成的生物吸附剂,用于去除铬(III)。
工业废水中重金属的存在对周围环境有害。重金属的生物吸附是处理工业废水的有效技术。这项研究工作是通过利用废鱼鳞作为生物吸附剂来去除铬(III)金属离子而进行的。采用不同的吸附剂剂量,溶液pH值和接触时间进行分批吸附过程。结果显示,在生物吸附剂剂量为0.8 g,溶液的pH为5,接触时间为90分钟,初始浓度为150 mg / l铬离子的情况下,最高的99.7518%铬(III)金属离子。吸附等温线数据与Langmuir等温线模型非常吻合,R 2 = 0.9998,q max = 18.3486 mg / g,R L= 0.00007325。还分析了伪第一动力学模型和第二动力学模型以描述吸附,发现该模型非常适合(R 2 = 1)吸附动力学。通过扫描电子显微镜,X射线光谱,傅里叶变换红外光谱和热分析研究了活化鱼鳞和含铬鱼鳞的表面特性,并与结果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号