当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.12.014 Liang-Qing Dong 1 , Li-Hua Peng 2 , Li-Jie Ma 1 , Dong-Bing Liu 3 , Shu Zhang 1 , Shu-Zhen Luo 2 , Jun-Hua Rao 2 , Hong-Wen Zhu 4 , Shuai-Xi Yang 1 , Shui-Jun Xi 1 , Min Chen 5 , Fan-Fan Xie 2 , Fu-Qiang Li 2 , Wen-Hui Li 2 , Chen Ye 2 , Li-Ya Lin 2 , Yu-Jue Wang 2 , Xiao-Ying Wang 1 , Da-Ming Gao 5 , Hu Zhou 4 , Huan-Ming Yang 6 , Jian Wang 6 , Shi-da Zhu 7 , Xiang-Dong Wang 8 , Ya Cao 9 , Jian Zhou 10 , Jia Fan 11 , Kui Wu 12 , Qiang Gao 10
Journal of Hepatology ( IF 26.8 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jhep.2019.12.014 Liang-Qing Dong 1 , Li-Hua Peng 2 , Li-Jie Ma 1 , Dong-Bing Liu 3 , Shu Zhang 1 , Shu-Zhen Luo 2 , Jun-Hua Rao 2 , Hong-Wen Zhu 4 , Shuai-Xi Yang 1 , Shui-Jun Xi 1 , Min Chen 5 , Fan-Fan Xie 2 , Fu-Qiang Li 2 , Wen-Hui Li 2 , Chen Ye 2 , Li-Ya Lin 2 , Yu-Jue Wang 2 , Xiao-Ying Wang 1 , Da-Ming Gao 5 , Hu Zhou 4 , Huan-Ming Yang 6 , Jian Wang 6 , Shi-da Zhu 7 , Xiang-Dong Wang 8 , Ya Cao 9 , Jian Zhou 10 , Jia Fan 11 , Kui Wu 12 , Qiang Gao 10
Affiliation
|
BACKGROUND AIMS
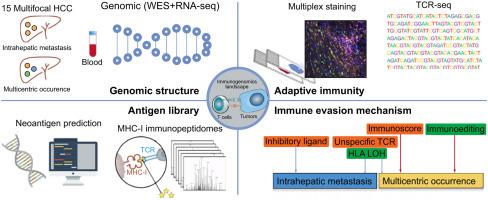
Multifocal tumors, developed either from intrahepatic metastasis (IM) or multicentric occurrence (MO), is a distinct feature of hepatocellular carcinoma (HCC). Immunogenomic characterization of multifocal HCC is important for understanding immune escape in different lesions and developing immunotherapy. METHODS
We combined whole exome/transcriptome sequencing, multiplex immunostaining, immunopeptidomes, T-cell receptor (TCR) sequencing and bioinformatic analyses of 47 tumors from 15 HCC patients with multifocal lesions. RESULTS
IM and MO demonstrated distinct clonal architecture, mutational spectrum and genetic susceptibility. The immune microenvironment also displayed spatiotemporal heterogeneity, such as less T cell and more M2 macrophage infiltration in IM and higher expression of inhibitory immune checkpoints in MO. Similar to mutational profiles, shared neoantigens and TCR repertoires among tumors from the same patients were abundant in IM but scarce in MO. Combining neoantigen prediction and immunopeptidomes identified T-cell specific neoepitopes and achieved a high verification rate in vitro. Immunoediting mainly occurred in MO but not IM, due to the relatively low immune infiltration. HLA LOH, identified in 17% of multifocal HCC, hampered the ability of MHC to present neoantigens, especially in IM. An integrated analysis of Immunoscore, Immunoediting, TCR clonality and HLA LOH of each tumor could stratify patients into two groups with high or low risk of recurrence (P=0.038). CONCLUSION
Our study comprehensively characterized the genetic structure, neoepitope landscape, T cell profile and immunoediting status that collectively shape tumor evolution, which may optimize personalized immunotherapies for multifocal HCC.
中文翻译:

多灶性肝细胞癌的异质免疫基因组特征和不同的逃逸机制
背景目的 多灶性肿瘤由肝内转移 (IM) 或多中心发生 (MO) 发展而来,是肝细胞癌 (HCC) 的一个明显特征。多灶性 HCC 的免疫基因组学特征对于了解不同病变中的免疫逃逸和开发免疫疗法很重要。方法我们结合了全外显子组/转录组测序、多重免疫染色、免疫肽组、T 细胞受体 (TCR) 测序和生物信息学分析,对来自 15 名具有多灶性病变的 HCC 患者的 47 个肿瘤进行了分析。结果 IM 和 MO 表现出不同的克隆结构、突变谱和遗传易感性。免疫微环境也表现出时空异质性,例如 IM 中较少的 T 细胞和更多的 M2 巨噬细胞浸润以及 MO 中抑制性免疫检查点的更高表达。与突变谱相似,来自同一患者的肿瘤之间共享的新抗原和 TCR 库在 IM 中很丰富,但在 MO 中很少。结合新抗原预测和免疫肽组鉴定了 T 细胞特异性新表位,并在体外实现了高验证率。由于免疫浸润相对较低,免疫编辑主要发生在 MO 而不是 IM。在 17% 的多灶性 HCC 中鉴定出 HLA LOH,阻碍了 MHC 呈递新抗原的能力,尤其是在 IM 中。对每个肿瘤的免疫评分、免疫编辑、TCR 克隆性和 HLA LOH 的综合分析可以将患者分为复发风险高或低的两组(P = 0.038)。结论 我们的研究全面表征了共同塑造肿瘤进化的遗传结构、新表位景观、T 细胞谱和免疫编辑状态,
更新日期:2020-05-01
中文翻译:

多灶性肝细胞癌的异质免疫基因组特征和不同的逃逸机制
背景目的 多灶性肿瘤由肝内转移 (IM) 或多中心发生 (MO) 发展而来,是肝细胞癌 (HCC) 的一个明显特征。多灶性 HCC 的免疫基因组学特征对于了解不同病变中的免疫逃逸和开发免疫疗法很重要。方法我们结合了全外显子组/转录组测序、多重免疫染色、免疫肽组、T 细胞受体 (TCR) 测序和生物信息学分析,对来自 15 名具有多灶性病变的 HCC 患者的 47 个肿瘤进行了分析。结果 IM 和 MO 表现出不同的克隆结构、突变谱和遗传易感性。免疫微环境也表现出时空异质性,例如 IM 中较少的 T 细胞和更多的 M2 巨噬细胞浸润以及 MO 中抑制性免疫检查点的更高表达。与突变谱相似,来自同一患者的肿瘤之间共享的新抗原和 TCR 库在 IM 中很丰富,但在 MO 中很少。结合新抗原预测和免疫肽组鉴定了 T 细胞特异性新表位,并在体外实现了高验证率。由于免疫浸润相对较低,免疫编辑主要发生在 MO 而不是 IM。在 17% 的多灶性 HCC 中鉴定出 HLA LOH,阻碍了 MHC 呈递新抗原的能力,尤其是在 IM 中。对每个肿瘤的免疫评分、免疫编辑、TCR 克隆性和 HLA LOH 的综合分析可以将患者分为复发风险高或低的两组(P = 0.038)。结论 我们的研究全面表征了共同塑造肿瘤进化的遗传结构、新表位景观、T 细胞谱和免疫编辑状态,










































 京公网安备 11010802027423号
京公网安备 11010802027423号