当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Post-Polymerization Functionalization of Halogen-Substituted Polyphosphinoboranes to Access Alkyne-Functionalized Derivatives.
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2019-12-27 , DOI: 10.1002/marc.201900468 Marius I Arz 1 , Alastair W Knights 1 , Ian Manners 1, 2
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2019-12-27 , DOI: 10.1002/marc.201900468 Marius I Arz 1 , Alastair W Knights 1 , Ian Manners 1, 2
Affiliation
|
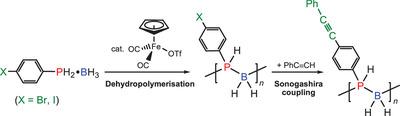
Catalytic dehydropolymerization of halogen-functionalized phosphine-boranes (4-X-C6 H4 )PH2 ⋅BH3 (1a: X = Br, 1b: X = I) with [CpFe(CO)2 (OTf)] at 100 °C provides convenient access to halogen-functionalized polyphosphinoboranes [(4-X-C6 H4 )PH-BH2 ]n (2a: X = Br, 2b: X = I). These polymers are useful precursors for post-polymerization functionalization, which is demonstrated by Sonogashira coupling under mild conditions to yield the alkynyl-functionalized polyphosphinoborane [(4-PhCC-C6 H4 )PH-BH2 ]n (3).
中文翻译:

卤素取代的聚膦硼烷的合成和聚合后的功能化,以得到炔烃官能化的衍生物。
卤素官能化的膦硼烷(4-X-C6 H4)PH2·BH3(1a:X = Br,1b:X = I)与[CpFe(CO)2(OTf)]的100°C催化脱氢反应提供了便利获得卤素官能化的聚膦硼烷[(4-X-C6 H4)PH-BH2] n(2a:X = Br,2b:X = I)。这些聚合物是用于聚合后官能化的有用前体,Sonogashira偶联在温和的条件下得到了证明,可得到炔基官能化的聚膦硼烷[(4-PhCC-C6H4)PH-BH2] n(3)。
更新日期:2019-12-27
中文翻译:

卤素取代的聚膦硼烷的合成和聚合后的功能化,以得到炔烃官能化的衍生物。
卤素官能化的膦硼烷(4-X-C6 H4)PH2·BH3(1a:X = Br,1b:X = I)与[CpFe(CO)2(OTf)]的100°C催化脱氢反应提供了便利获得卤素官能化的聚膦硼烷[(4-X-C6 H4)PH-BH2] n(2a:X = Br,2b:X = I)。这些聚合物是用于聚合后官能化的有用前体,Sonogashira偶联在温和的条件下得到了证明,可得到炔基官能化的聚膦硼烷[(4-PhCC-C6H4)PH-BH2] n(3)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号