当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Coordinate Formal Cobalt(0), Iron(0), and Manganese(0) Complexes with Persistent Carbene and Alkene Ligation.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.accounts.9b00492 Yang Liu 1 , Jun Cheng 1 , Liang Deng 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.accounts.9b00492 Yang Liu 1 , Jun Cheng 1 , Liang Deng 1
Affiliation
|
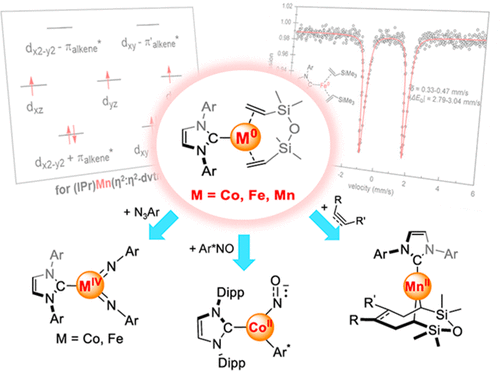
Low-coordinate transition-metal species, i.e., metal species with coordination numbers of less than 4, represent a category of ubiquitous reactive intermediates in metal-catalyzed reactions that take place in solution, in metalloenzymes, on supported nanomaterials and single-atom catalysts, and so on. While reactive intermediates are usually transient and hard to isolate, which makes detailed investigation challenging, molecular representatives of low-coordinate transition-metal intermediates can be synthesized by the judicious use of supporting ligands, allowing detailed study of their inherent chemical and physical properties. By the use of bulky nitrogen- and oxygen-based anionic ligands, plenty of three- and two-coordinate group 4-10 metal complexes with the oxidation states of the metal centers being +3, + 2, and +1 have been prepared and subjected to extensive study. Much less known are low-coordinate zero-valent metal complexes, and knowledge about them had been restricted to group 10 metal complexes until very recently. In this Account, we summarize the studies of the synthesis, spectroscopic features, electronic structures, and reactivities of three-coordinate formal cobalt(0), iron(0), and manganese(0) complexes with persistent carbene and alkene ligation. The introduction of the π-accepting alkene ligands 1,3-divinyl-1,1,3,3-tetramethyldisiloxane (dvtms) and vinyltrimethylsilane (vtms) into the reduction reactions of MCl2 (M = Co, Fe, Mn) with persistent carbenes and alkaline metals effectively suppresses ligand C-H bond activation reactions, leading to the successful preparation of three-coordinate formal cobalt(0), iron(0), and manganese(0) complexes LM(η2:η2-dvtms), LM(η2-vtms)2, and L2M(η2-vtms) (M = Co, Fe, Mn; L = N-heterocyclic carbene (NHC), cyclic (alkyl)(amino)carbene (cAAC)). These three-coordinate metal complexes feature pronounced back-donation from the filled metal 3d orbitals to the alkene π* orbital(s), resulting in electronic configurations of (dxy+πalkene*)2(dx2-y2+π'alkene*)2(dz2,dxz,dyz)n (the coordination plane was chosen as the xy plane; n = 5, 4, and 3 for Co, Fe, and Mn, respectively) for the bis(alkene) complexes LM(η2:η2-dvtms) and LM(η2-vtms)2. The alkene ligands in the low-coordinate formal zero-valent metal complexes are amenable to undergo ligand-exchange reactions with better π-accepting ligands. In reactions with organic azides, hydrosilanes, nitrosoarenes, alkynes, etc., the alkene ligands dissociate from the metal coordination sphere, and three-coordinate formal zero-valent metal complexes function as synthons of LnM0 (L = NHC, cAAC; n = 1, 2; M = Co, Fe, Mn) to perform redox reactions with these substrates, affording divalent and tetravalent cobalt, iron, or manganese complexes. These electronic structure and reactivity features hint at the potential of low-coordinate zero-valent group 7-9 metal complexes for the development of new 3d metal catalysts and magnetic materials.
中文翻译:

具有持久碳和烯键合的三坐标形式钴(0),铁(0)和锰(0)配合物。
低配位的过渡金属物种,即配位数小于4的金属物种,代表了在溶液中,金属酶中,负载型纳米材料和单原子催化剂上发生的金属催化反应中普遍存在的反应中间体,等等。尽管反应性中间体通常是瞬态的且难以分离,这使详细研究变得困难,但低配位过渡金属中间体的分子代表可以通过明智地使用支持配体来合成,从而可以对其内在的化学和物理性质进行详细研究。通过使用庞大的基于氮和氧的阴离子配体,大量的三配位和二配位的4-10族金属配合物,金属中心的氧化态为+ 3,+ 2 和+1已准备好并接受了广泛的研究。低配位的零价金属络合物鲜为人知,直到最近,有关它们的知识还仅限于第10组金属络合物。在此报告中,我们总结了具有持久卡宾和烯烃键合的三坐标形式钴(0),铁(0)和锰(0)配合物的合成,光谱特征,电子结构和反应性的研究。将π接受链烯配体1,3-二乙烯基-1,1,3,3-四甲基二硅氧烷(dvtms)和乙烯基三甲基硅烷(vtms)引入MCl2(M = Co,Fe,Mn)与持久性卡宾的还原反应中和碱金属有效抑制配体CH键活化反应,从而成功制备了三配位形式的钴(0),铁(0),和锰(0)络合物LM(η2:η2-dvtms),LM(η2-vtms)2和L2M(η2-vtms)(M = Co,Fe,Mn; L = N杂环卡宾(NHC),环状(烷基)(氨基)卡宾(cAAC))。这些三配位金属络合物的特征是从填充金属3d轨道到烯烃π*轨道有明显的反向捐赠,导致(dxy +π烯烃*)2(dx2-y2 +π'烯烃*)2的电子构型(dz2,dxz,dyz)n(将协调平面选择为xy平面;对于Co,Fe和Mn,n分别为5、4和3)双(烯烃)配合物LM(η2:η2- dvtms)和LM(η2-vtms)2。低配位形式零价金属络合物中的烯烃配体易于与更好的π接受配体进行配体交换反应。与有机叠氮化物,氢硅烷,亚硝基芳烃,炔烃等反应时,烯烃配体从金属配位球上解离,三价形式的零价金属络合物作为LnM0的合成子(L = NHC,cAAC; n = 1,2; M = Co,Fe,Mn)进行氧化还原反应用这些底物,得到二价和四价的钴,铁或锰配合物。这些电子结构和反应性特征暗示了低配位的零价7-9族金属配合物在开发新型3d金属催化剂和磁性材料方面的潜力。
更新日期:2019-12-27
中文翻译:

具有持久碳和烯键合的三坐标形式钴(0),铁(0)和锰(0)配合物。
低配位的过渡金属物种,即配位数小于4的金属物种,代表了在溶液中,金属酶中,负载型纳米材料和单原子催化剂上发生的金属催化反应中普遍存在的反应中间体,等等。尽管反应性中间体通常是瞬态的且难以分离,这使详细研究变得困难,但低配位过渡金属中间体的分子代表可以通过明智地使用支持配体来合成,从而可以对其内在的化学和物理性质进行详细研究。通过使用庞大的基于氮和氧的阴离子配体,大量的三配位和二配位的4-10族金属配合物,金属中心的氧化态为+ 3,+ 2 和+1已准备好并接受了广泛的研究。低配位的零价金属络合物鲜为人知,直到最近,有关它们的知识还仅限于第10组金属络合物。在此报告中,我们总结了具有持久卡宾和烯烃键合的三坐标形式钴(0),铁(0)和锰(0)配合物的合成,光谱特征,电子结构和反应性的研究。将π接受链烯配体1,3-二乙烯基-1,1,3,3-四甲基二硅氧烷(dvtms)和乙烯基三甲基硅烷(vtms)引入MCl2(M = Co,Fe,Mn)与持久性卡宾的还原反应中和碱金属有效抑制配体CH键活化反应,从而成功制备了三配位形式的钴(0),铁(0),和锰(0)络合物LM(η2:η2-dvtms),LM(η2-vtms)2和L2M(η2-vtms)(M = Co,Fe,Mn; L = N杂环卡宾(NHC),环状(烷基)(氨基)卡宾(cAAC))。这些三配位金属络合物的特征是从填充金属3d轨道到烯烃π*轨道有明显的反向捐赠,导致(dxy +π烯烃*)2(dx2-y2 +π'烯烃*)2的电子构型(dz2,dxz,dyz)n(将协调平面选择为xy平面;对于Co,Fe和Mn,n分别为5、4和3)双(烯烃)配合物LM(η2:η2- dvtms)和LM(η2-vtms)2。低配位形式零价金属络合物中的烯烃配体易于与更好的π接受配体进行配体交换反应。与有机叠氮化物,氢硅烷,亚硝基芳烃,炔烃等反应时,烯烃配体从金属配位球上解离,三价形式的零价金属络合物作为LnM0的合成子(L = NHC,cAAC; n = 1,2; M = Co,Fe,Mn)进行氧化还原反应用这些底物,得到二价和四价的钴,铁或锰配合物。这些电子结构和反应性特征暗示了低配位的零价7-9族金属配合物在开发新型3d金属催化剂和磁性材料方面的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号