当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Hydrogen Bonding in Phase Change Materials
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.cgd.9b01541 Karolina Matuszek 1 , R. Vijayaraghavan 1 , Mega Kar 1 , Douglas R. MacFarlane 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.cgd.9b01541 Karolina Matuszek 1 , R. Vijayaraghavan 1 , Mega Kar 1 , Douglas R. MacFarlane 1
Affiliation
|
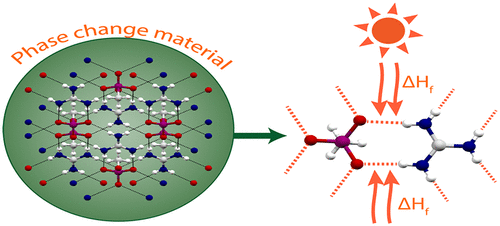
Phase change materials (PCMs), which melt in the temperature range of 100–230 °C, are a promising alternative for the storage of thermal energy. In this range, large amounts of energy available from solar-thermal, or other forms of renewable heat, can be stored and applied to domestic or industrial processes, or to an organic Rankine cycle (ORC) engine to generate electricity. The amount of energy absorbed is related to the latent heat of fusion (ΔHf) and is often connected to the extent of hydrogen bonding in the PCM. Herein, we report fundamental studies, including crystal structure and Hirshfeld surface analysis, of a family of guanidinium organic salts that exhibit high values of ΔHf , demonstrating that the presence and strength of H-bonds between ions play a key role in this property.
中文翻译:

氢键在相变材料中的作用
相变材料(PCM)在100–230°C的温度范围内熔化,是一种有前途的热能存储替代品。在此范围内,可以存储来自太阳热能或其他形式的可再生热能的大量能量,并将其应用于家庭或工业过程,或应用于有机朗肯循环(ORC)发动机以发电。吸收的能量与熔化潜热(ΔH f)有关,并且通常与PCM中的氢键结合程度有关。在此,我们报告了对ΔH f值较高的胍类有机盐家族的基础研究,包括晶体结构和Hirshfeld表面分析。 ,表明离子之间氢键的存在和强度在该特性中起关键作用。
更新日期:2020-01-10
中文翻译:

氢键在相变材料中的作用
相变材料(PCM)在100–230°C的温度范围内熔化,是一种有前途的热能存储替代品。在此范围内,可以存储来自太阳热能或其他形式的可再生热能的大量能量,并将其应用于家庭或工业过程,或应用于有机朗肯循环(ORC)发动机以发电。吸收的能量与熔化潜热(ΔH f)有关,并且通常与PCM中的氢键结合程度有关。在此,我们报告了对ΔH f值较高的胍类有机盐家族的基础研究,包括晶体结构和Hirshfeld表面分析。 ,表明离子之间氢键的存在和强度在该特性中起关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号