Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple catalytic activities of human 17β-hydroxysteroid dehydrogenase type 7 respond differently to inhibitors.
Biochimie ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.biochi.2019.12.012 Terenzio Ferrante 1 , Salvatore Adinolfi 1 , Giulia D'Arrigo 1 , Donald Poirier 2 , Martina Daga 1 , Marco Lucio Lolli 1 , Gianni Balliano 1 , Francesca Spyrakis 1 , Simonetta Oliaro-Bosso 1
Biochimie ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1016/j.biochi.2019.12.012 Terenzio Ferrante 1 , Salvatore Adinolfi 1 , Giulia D'Arrigo 1 , Donald Poirier 2 , Martina Daga 1 , Marco Lucio Lolli 1 , Gianni Balliano 1 , Francesca Spyrakis 1 , Simonetta Oliaro-Bosso 1
Affiliation
|
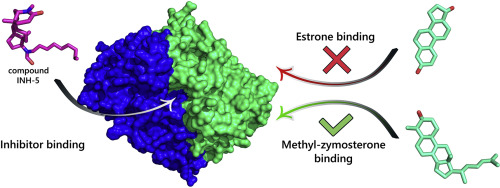
Cholesterol biosynthesis is a multistep process in mammals that includes the aerobic removal of three methyl groups from the intermediate lanosterol, one from position 14 and two from position 4. During the demethylations at position 4, a 3-ketosteroid reductase catalyses the conversion of both 4-methylzymosterone and zymosterone to 4-methylzymosterol and zymosterol, respectively, restoring the alcoholic function of lanosterol, which is also maintained in cholesterol. Unlike other eukaryotes, mammals also use the same enzyme as an estrone reductase that can transform estrone (E1) into estradiol (E2). This enzyme, named 17β-hydroxysteroid dehydrogenase type 7 (HSD17B7), is therefore a multifunctional protein in mammals, and one that belongs to both the HSD17B family, which is involved in steroid-hormone metabolism, and to the family of post-squalene cholesterol biosynthesis enzymes. In the present study, a series of known inhibitors of human HSD17B7's E1-reductase activity have been assayed for potential inhibition against 3-ketosteroid reductase activity. Surprisingly, the assayed compounds lost their inhibition activity when tested in HepG2 cells that were incubated with radiolabelled acetate and against the recombinant overexpressed human enzyme incubated with 4-methylzymosterone (both radiolabelled and not). Preliminary kinetic analyses suggest a mixed or non-competitive inhibition on the E1-reductase activity, which is in agreement with Molecular Dynamics simulations. These results raise questions about the mechanism(s) of action of these possible inhibitors, the enzyme dynamic regulation and the interplay between the two activities.
中文翻译:

7型人17β-羟类固醇脱氢酶的多种催化活性对抑制剂的反应不同。
胆固醇的生物合成在哺乳动物中是一个多步骤过程,包括有氧从中间羊毛甾醇中去除三个甲基,一个从位置14去除,两个从位置4去除。在位置4的去甲基化过程中,3-酮类固醇还原酶催化两个4的转化。 -甲基-甲基雌甾烷酮和zymosterone分别变为4-甲基zymosterol和zymosterol,恢复了在胆固醇中也保持的羊毛甾醇的酒精功能。与其他真核生物不同,哺乳动物还使用与雌酮还原酶相同的酶,该酶可以将雌酮(E1)转化为雌二醇(E2)。因此,这种名为17β-羟基类固醇脱氢酶7型(HSD17B7)的酶是哺乳动物中的一种多功能蛋白,并且属于与类固醇激素代谢有关的HSD17B家族,以及角鲨烯后胆固醇生物合成酶家族。在本研究中,已测定了一系列已知的人类HSD17B7 E1-还原酶活性抑制剂对3-酮类固醇还原酶活性的潜在抑制作用。出乎意料的是,当在用放射性标记的乙酸盐孵育的HepG2细胞中和用4-甲基zymosterone孵育的重组过表达的人酶中(无论是放射性标记的还是未标记的),被测化合物均失去了抑制活性。初步的动力学分析表明,对E1还原酶活性存在混合或非竞争性抑制作用,这与分子动力学模拟一致。这些结果引起了关于这些可能的抑制剂的作用机理,酶动态调节以及两种活性之间相互作用的问题。
更新日期:2019-12-27
中文翻译:

7型人17β-羟类固醇脱氢酶的多种催化活性对抑制剂的反应不同。
胆固醇的生物合成在哺乳动物中是一个多步骤过程,包括有氧从中间羊毛甾醇中去除三个甲基,一个从位置14去除,两个从位置4去除。在位置4的去甲基化过程中,3-酮类固醇还原酶催化两个4的转化。 -甲基-甲基雌甾烷酮和zymosterone分别变为4-甲基zymosterol和zymosterol,恢复了在胆固醇中也保持的羊毛甾醇的酒精功能。与其他真核生物不同,哺乳动物还使用与雌酮还原酶相同的酶,该酶可以将雌酮(E1)转化为雌二醇(E2)。因此,这种名为17β-羟基类固醇脱氢酶7型(HSD17B7)的酶是哺乳动物中的一种多功能蛋白,并且属于与类固醇激素代谢有关的HSD17B家族,以及角鲨烯后胆固醇生物合成酶家族。在本研究中,已测定了一系列已知的人类HSD17B7 E1-还原酶活性抑制剂对3-酮类固醇还原酶活性的潜在抑制作用。出乎意料的是,当在用放射性标记的乙酸盐孵育的HepG2细胞中和用4-甲基zymosterone孵育的重组过表达的人酶中(无论是放射性标记的还是未标记的),被测化合物均失去了抑制活性。初步的动力学分析表明,对E1还原酶活性存在混合或非竞争性抑制作用,这与分子动力学模拟一致。这些结果引起了关于这些可能的抑制剂的作用机理,酶动态调节以及两种活性之间相互作用的问题。









































 京公网安备 11010802027423号
京公网安备 11010802027423号