当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
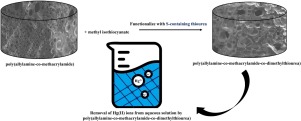
Removal of Hg(II) ions from aqueous solution by poly(allylamine-co-methacrylamide-co-dimethylthiourea)
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jiec.2019.12.023 Mi Young Kim , Haein Seo , Tai Gyu Lee
Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jiec.2019.12.023 Mi Young Kim , Haein Seo , Tai Gyu Lee
|
Abstract In this study, poly(allylamine-co-methacrylamide-co-dimethylthiourea), a chelating polymer containing amino groups and thiourea, was synthesized and tested for its capacity to remove mercury ions (Hg2+) from aqueous solutions. The structure of the prepared polymer was analyzed and characterized by FTIR and FE-SEM. The effects of various parameters such as pH, contact time, and initial Hg concentration on Hg(II) removal were studied. The Hg(II) adsorption reached maximum capacity at equilibrium within 1 h. The Hg ions were adsorbed for 4 h at pH 7. The highest Hg(II) adsorption was obtained at pH 7 and 400 mg L−1. The experimental data were fitted to the pseudo-second-order model using kinetic modeling, indicating that the mechanism was chemisorption. In addition, the experimental data were fitted to the Langmuir isotherm model, thus demonstrating that the Hg(II) adsorption process occurred through the formation of a monolayer.
中文翻译:

聚(烯丙胺-共-甲基丙烯酰胺-共-二甲基硫脲)从水溶液中去除Hg(II)离子
摘要 本研究合成了聚(烯丙胺-共-甲基丙烯酰胺-共-二甲基硫脲),一种含有氨基和硫脲的螯合聚合物,并测试了其从水溶液中去除汞离子 (Hg2+) 的能力。通过FTIR和FE-SEM对制备的聚合物进行了结构分析和表征。研究了各种参数(如 pH 值、接触时间和初始 Hg 浓度)对 Hg(II) 去除的影响。Hg(II) 吸附在 1 小时内达到平衡时的最大容量。Hg 离子在 pH 7 下吸附 4 小时。在 pH 7 和 400 mg L-1 下获得最高的 Hg(II) 吸附。使用动力学模型将实验数据拟合到伪二级模型,表明其机理是化学吸附。此外,实验数据符合朗缪尔等温线模型,
更新日期:2020-04-01
中文翻译:

聚(烯丙胺-共-甲基丙烯酰胺-共-二甲基硫脲)从水溶液中去除Hg(II)离子
摘要 本研究合成了聚(烯丙胺-共-甲基丙烯酰胺-共-二甲基硫脲),一种含有氨基和硫脲的螯合聚合物,并测试了其从水溶液中去除汞离子 (Hg2+) 的能力。通过FTIR和FE-SEM对制备的聚合物进行了结构分析和表征。研究了各种参数(如 pH 值、接触时间和初始 Hg 浓度)对 Hg(II) 去除的影响。Hg(II) 吸附在 1 小时内达到平衡时的最大容量。Hg 离子在 pH 7 下吸附 4 小时。在 pH 7 和 400 mg L-1 下获得最高的 Hg(II) 吸附。使用动力学模型将实验数据拟合到伪二级模型,表明其机理是化学吸附。此外,实验数据符合朗缪尔等温线模型,



























 京公网安备 11010802027423号
京公网安备 11010802027423号