当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Determination and Thermodynamic Correlation of 7-Amino-4-Methylcoumarin Solubility in Various Cosolvency Mixtures at (278.15–323.15) K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jced.9b00873 Yuli Shi 1, 2 , Haojian Zhang 1 , Xiaomin Hong 1 , Xiaodong Wang 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jced.9b00873 Yuli Shi 1, 2 , Haojian Zhang 1 , Xiaomin Hong 1 , Xiaodong Wang 2
Affiliation
|
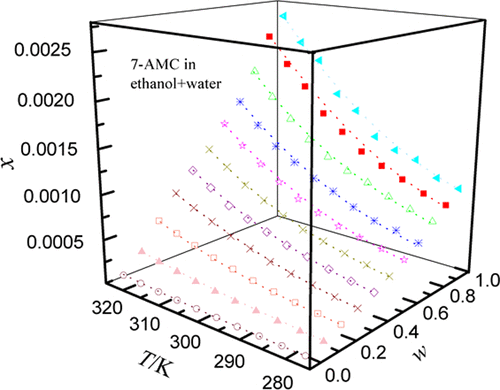
Four solvents (ethanol, isopropanol, ethylene glycol (EG), and N,N-dimethylformamide (DMF)) that can be mixed with water in any ratio were selected to determine the dissolution performance of 7-amino-4-methylcoumarin by the classical shake-flask method. The measured temperature range began at 278.15 K and ended at 303.15 K, and the pressure environment was controlled at standard atmospheric pressure (101.1 kPa). Results reveal that with the addition of organic solvents, the solubilization effect of 7-amino-4-methylcoumarin was very significant and the larger the amount of addition, the more obvious was the effect of solubilization. Not only that, the temperature change had a non-negligible effect on the dispersion of 7-amino-4-methylcoumarin; the temperature increased monotonically, and the better was the dissolution. When the external conditions were kept constant, the addition of DMF made the solubilization effect of 7-amino-4-methylcoumarin most obvious among all organic solvents used. This study involved three models, including the Jouyban–Acree model and its two variants (van’t Hoff–Jouyban–Acree model and Apelblat–Jouyban–Acree model), which were used to correlate the solubility data of 7-amino-4-methylcoumarin in aqueous cosolvent mixtures. The relative average deviation (RAD) and root-mean-square deviation (RMSD) reaches to 3.47 × 10–2 and 1.79 × 10–3 rooting in the van’t Hoff–Jouyban–Acree model. The relevant parameters obtained through model calculation and experimental means are essential for product synthesis, separation, and purification processes.
中文翻译:

在(278.15–323.15)K下不同溶解度混合物中7-氨基-4-甲基香豆素溶解度的实验测定和热力学相关性
四种溶剂(乙醇,异丙醇,乙二醇(EG)和N,N选择可以与水以任何比例混合的-二甲基甲酰胺(DMF),以通过经典摇瓶法测定7-氨基-4-甲基香豆素的溶解性能。测得的温度范围开始于278.15 K,结束于303.15 K,压力环境被控制在标准大气压(101.1 kPa)。结果表明,在添加有机溶剂的情况下,7-氨基-4-甲基香豆素的增溶效果非常显着,添加量越大,增溶效果越明显。不仅如此,温度变化对7-氨基-4-甲基香豆素的分散性影响不可忽略。温度单调升高,且溶解度更好。当外部条件保持不变时,在所有使用的有机溶剂中,DMF的添加使7-氨基-4-甲基香豆素的增溶作用最明显。这项研究涉及三个模型,包括Jouyban–Acree模型及其两个变体(van't Hoff–Jouyban–Acree模型和Apelblat–Jouyban–Acree模型),这些模型用于关联7-氨基-4-的溶解度数据。在水性助溶剂混合物中的甲基香豆素。相对平均偏差(RAD)和均方根偏差(RMSD)达到3.47×10–2和1.79×10 –3根植于van't Hoff–Jouyban–Acree模型。通过模型计算和实验手段获得的相关参数对于产品合成,分离和纯化过程至关重要。
更新日期:2019-12-27
中文翻译:

在(278.15–323.15)K下不同溶解度混合物中7-氨基-4-甲基香豆素溶解度的实验测定和热力学相关性
四种溶剂(乙醇,异丙醇,乙二醇(EG)和N,N选择可以与水以任何比例混合的-二甲基甲酰胺(DMF),以通过经典摇瓶法测定7-氨基-4-甲基香豆素的溶解性能。测得的温度范围开始于278.15 K,结束于303.15 K,压力环境被控制在标准大气压(101.1 kPa)。结果表明,在添加有机溶剂的情况下,7-氨基-4-甲基香豆素的增溶效果非常显着,添加量越大,增溶效果越明显。不仅如此,温度变化对7-氨基-4-甲基香豆素的分散性影响不可忽略。温度单调升高,且溶解度更好。当外部条件保持不变时,在所有使用的有机溶剂中,DMF的添加使7-氨基-4-甲基香豆素的增溶作用最明显。这项研究涉及三个模型,包括Jouyban–Acree模型及其两个变体(van't Hoff–Jouyban–Acree模型和Apelblat–Jouyban–Acree模型),这些模型用于关联7-氨基-4-的溶解度数据。在水性助溶剂混合物中的甲基香豆素。相对平均偏差(RAD)和均方根偏差(RMSD)达到3.47×10–2和1.79×10 –3根植于van't Hoff–Jouyban–Acree模型。通过模型计算和实验手段获得的相关参数对于产品合成,分离和纯化过程至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号