当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weak Intermolecular CH···N Hydrogen Bonding: Determination of 13CH-15N Hydrogen-Bond Mediated J Couplings by Solid-State NMR Spectroscopy and First-Principles Calculations.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpca.9b10726 Amy L Webber 1 , Jonathan R Yates 2 , Miri Zilka 1 , Simone Sturniolo 3 , Anne-Christine Uldry 4 , Emily K Corlett 1 , Chris J Pickard 5, 6 , Marta Pérez-Torralba 7 , M Angeles Garcia 7 , Dolores Santa Maria 7 , Rosa M Claramunt 7 , Steven P Brown 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpca.9b10726 Amy L Webber 1 , Jonathan R Yates 2 , Miri Zilka 1 , Simone Sturniolo 3 , Anne-Christine Uldry 4 , Emily K Corlett 1 , Chris J Pickard 5, 6 , Marta Pérez-Torralba 7 , M Angeles Garcia 7 , Dolores Santa Maria 7 , Rosa M Claramunt 7 , Steven P Brown 1
Affiliation
|
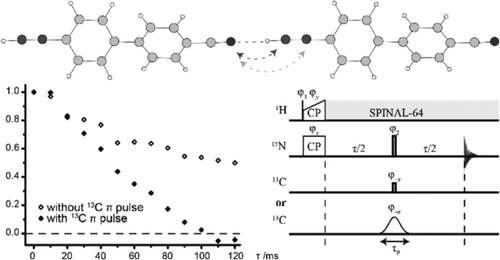
Weak hydrogen bonds are increasingly hypothesized to play key roles in a wide range of chemistry from catalysis to gelation to polymer structure. Here, 15N/13C spin-echo magic-angle spinning (MAS) solid-state nuclear magnetic resonance (NMR) experiments are applied to "view" intermolecular CH···N hydrogen bonding in two selectively labeled organic compounds, 4-[15N] cyano-4'-[13C2] ethynylbiphenyl (1) and [15N3,13C6]-2,4,6-triethynyl-1,3,5-triazine (2). The synthesis of 2-15N3,13C6 is reported here for the first time via a multistep procedure, where the key element is the reaction of [15N3]-2,4,6-trichloro-1,3,5-triazine (5) with [13C2]-[(trimethylsilyl)ethynyl]zinc chloride (8) to afford its immediate precursor [15N3,13C6]-2,4,6-tris[(trimethylsilyl)ethynyl]-1,3,5-triazine (9). Experimentally determined hydrogen-bond-mediated 2hJCN couplings (4.7 ± 0.4 Hz (1) and 4.1 ± 0.3 Hz (2)) are compared with density functional theory (DFT) gauge-including projector augmented wave (GIPAW) calculations, whereby species-independent coupling values 2hKCN (29.0 × 1019 kg m-2 s-2 A-2 (1) and 27.9 × 1019 kg m-2 s-2 A-2 (2)) quantitatively demonstrate the J couplings for these "weak" CH···N hydrogen bonds to be of a similar magnitude to those for conventionally observed NH···O hydrogen-bonding interactions in uracil (2hKNO: 28.1 and 36.8 × 1019 kg m-2 s-2 A-2). Moreover, the GIPAW calculations show a clear correlation between increasing 2hJCN (and 3hJCN) coupling and reducing C(H)···N and H···N hydrogen-bonding distances, with the Fermi contact term accounting for at least 98% of the isotropic 2hJCN coupling.
中文翻译:

弱分子间CH···N氢键键合:通过固态NMR光谱和第一性原理计算确定13CH-15N氢键介导的J偶联。
人们越来越认为,弱氢键在从催化到胶凝再到聚合物结构的广泛化学过程中起着关键作用。在这里,将15N / 13C自旋回波魔角旋转(MAS)固态核磁共振(NMR)实验用于“观察”在两个选择性标记的有机化合物4- [15N中的分子间CH···N氢键]氰基-4'-[13C2]乙炔基联苯(1)和[15N3,13C6] -2,4,6-三乙炔基-1,3,5-三嗪(2)。2-15N3,13C6的合成首次通过多步程序进行了报道,其中关键要素是[15N3] -2,4,6-三氯-1,3,5-三嗪的反应(5)用[13C2]-[((三甲基甲硅烷基)乙炔基]氯化锌(8)提供其直接前体[15N3,13C6] -2,4,6-三[[(三甲基甲硅烷基)乙炔基] -1,3,5-三嗪(9 )。将实验确定的氢键介导的2hJCN耦合(4.7±0.4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规-包括投影仪增强波(GIPAW)计算进行比较,从而与物种无关耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH·的J耦合··N氢键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规(包括投影仪增强波(GIPAW))进行了比较,从而与物种无关的耦合值2hKCN(29.0×1019 kg m-2 s -2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH···N氢键的J偶联量与常规观察到的在尿嘧啶中的NH···O氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规(包括投影仪增强波(GIPAW))进行了比较,从而与物种无关的耦合值2hKCN(29.0×1019 kg m-2 s -2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH···N氢键的J偶联量与在尿嘧啶中常规观察到的NH···O氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将3 Hz(2))与密度泛函理论(DFT)压力计(包括投影仪增强波(GIPAW))进行比较,从而独立于物种的耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量地证明了这些“弱” CH···N氢键的J偶联量与常规观察到的NH···相似。尿嘧啶中的氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将3 Hz(2))与密度泛函理论(DFT)压力计(包括投影仪增强波(GIPAW))进行比较,从而独立于物种的耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量地证明了这些“弱” CH···N氢键的J偶联量与常规观察到的NH···相似。尿嘧啶中的氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2)定量证明了这些“弱” CH···N氢的J偶联键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2)定量证明了这些“弱” CH···N氢的J偶联键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。8×1019公斤m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。8×1019公斤m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。
更新日期:2020-01-10
中文翻译:

弱分子间CH···N氢键键合:通过固态NMR光谱和第一性原理计算确定13CH-15N氢键介导的J偶联。
人们越来越认为,弱氢键在从催化到胶凝再到聚合物结构的广泛化学过程中起着关键作用。在这里,将15N / 13C自旋回波魔角旋转(MAS)固态核磁共振(NMR)实验用于“观察”在两个选择性标记的有机化合物4- [15N中的分子间CH···N氢键]氰基-4'-[13C2]乙炔基联苯(1)和[15N3,13C6] -2,4,6-三乙炔基-1,3,5-三嗪(2)。2-15N3,13C6的合成首次通过多步程序进行了报道,其中关键要素是[15N3] -2,4,6-三氯-1,3,5-三嗪的反应(5)用[13C2]-[((三甲基甲硅烷基)乙炔基]氯化锌(8)提供其直接前体[15N3,13C6] -2,4,6-三[[(三甲基甲硅烷基)乙炔基] -1,3,5-三嗪(9 )。将实验确定的氢键介导的2hJCN耦合(4.7±0.4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规-包括投影仪增强波(GIPAW)计算进行比较,从而与物种无关耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH·的J耦合··N氢键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规(包括投影仪增强波(GIPAW))进行了比较,从而与物种无关的耦合值2hKCN(29.0×1019 kg m-2 s -2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH···N氢键的J偶联量与常规观察到的在尿嘧啶中的NH···O氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将4 Hz(1)和4.1±0.3 Hz(2))与密度泛函理论(DFT)量规(包括投影仪增强波(GIPAW))进行了比较,从而与物种无关的耦合值2hKCN(29.0×1019 kg m-2 s -2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量证明了这些“弱” CH···N氢键的J偶联量与在尿嘧啶中常规观察到的NH···O氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将3 Hz(2))与密度泛函理论(DFT)压力计(包括投影仪增强波(GIPAW))进行比较,从而独立于物种的耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量地证明了这些“弱” CH···N氢键的J偶联量与常规观察到的NH···相似。尿嘧啶中的氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。将3 Hz(2))与密度泛函理论(DFT)压力计(包括投影仪增强波(GIPAW))进行比较,从而独立于物种的耦合值2hKCN(29.0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2))定量地证明了这些“弱” CH···N氢键的J偶联量与常规观察到的NH···相似。尿嘧啶中的氢键相互作用(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2)定量证明了这些“弱” CH···N氢的J偶联键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。0×1019 kg m-2 s-2 A-2(1)和27.9×1019 kg m-2 s-2 A-2(2)定量证明了这些“弱” CH···N氢的J偶联键的大小与尿嘧啶中常规观察到的NH··O氢键相互作用的大小相似(2hKNO:28.1和36.8×1019 kg m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。8×1019公斤m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。8×1019公斤m-2 s-2 A-2)。此外,GIPAW计算结果表明,增加2hJCN(和3hJCN)耦合与减少C(H)··N和H··N氢键合距离之间存在明显的相关性,费米接触项至少占到98%的氢键。各向同性2hJCN耦合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号