当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of the Enabling Route for Glecaprevir via Ring-Closing Metathesis
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.oprd.9b00469 Russell D. Cink 1 , Kirill A. Lukin 1 , Richard D. Bishop 1 , Gang Zhao 1 , Matthew J. Pelc 1 , Timothy B. Towne 1 , Bradley D. Gates 1 , Matthew M. Ravn 1 , David R. Hill 1 , Chen Ding 1 , Steven C. Cullen 1 , Jianzhang Mei 1 , M. Robert Leanna 1 , Jeremy Henle 1 , José G. Napolitano 1 , Nandkishor K. Nere 1 , Shuang Chen 1 , Ahmad Sheikh 1 , Jeffrey M. Kallemeyn 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.oprd.9b00469 Russell D. Cink 1 , Kirill A. Lukin 1 , Richard D. Bishop 1 , Gang Zhao 1 , Matthew J. Pelc 1 , Timothy B. Towne 1 , Bradley D. Gates 1 , Matthew M. Ravn 1 , David R. Hill 1 , Chen Ding 1 , Steven C. Cullen 1 , Jianzhang Mei 1 , M. Robert Leanna 1 , Jeremy Henle 1 , José G. Napolitano 1 , Nandkishor K. Nere 1 , Shuang Chen 1 , Ahmad Sheikh 1 , Jeffrey M. Kallemeyn 1
Affiliation
|
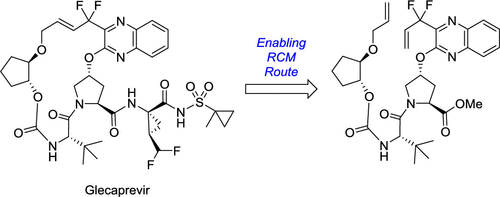
Glecaprevir was identified as a potent HCV NS3/4A protease inhibitor, and an enabling synthesis was required to support the preclinical evaluation and subsequent Phase I clinical trials. The enabling route to glecaprevir was established through further development of the medicinal chemistry route. The key steps in the synthesis involved a ring-closing metathesis (RCM) reaction to form the 18-membered macrocycle and a challenging fluorination step to form a key amino acid. The enabling route was successfully used to produce 41 kg of glecaprevir, sufficient to support the preclinical evaluation and early clinical development.
中文翻译:

通过环复分解开发格列卡韦的赋能途径
Glecaprevir被鉴定为有效的HCV NS3 / 4A蛋白酶抑制剂,需要进行有效的合成以支持临床前评估和随后的I期临床试验。通过进一步发展药物化学途径,确立了格列卡韦的赋能途径。合成中的关键步骤涉及闭环复分解(RCM)反应以形成18元大环,而挑战性的氟化步骤则是形成关键氨基酸。该成功途径已成功用于生产41千克格列卡韦韦,足以支持临床前评估和早期临床开发。
更新日期:2020-01-15
中文翻译:

通过环复分解开发格列卡韦的赋能途径
Glecaprevir被鉴定为有效的HCV NS3 / 4A蛋白酶抑制剂,需要进行有效的合成以支持临床前评估和随后的I期临床试验。通过进一步发展药物化学途径,确立了格列卡韦的赋能途径。合成中的关键步骤涉及闭环复分解(RCM)反应以形成18元大环,而挑战性的氟化步骤则是形成关键氨基酸。该成功途径已成功用于生产41千克格列卡韦韦,足以支持临床前评估和早期临床开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号