当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Syntheses of 4H-3,1-Benzoxazines via Catalytic Asymmetric Chlorocyclization of o-Vinylanilides.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.joc.9b02395 Qinxia Xie 1 , Hai-Jiao Long 1 , Qiong-Yin Zhang 1 , Pei Tang 1, 2 , Jun Deng 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.joc.9b02395 Qinxia Xie 1 , Hai-Jiao Long 1 , Qiong-Yin Zhang 1 , Pei Tang 1, 2 , Jun Deng 1
Affiliation
|
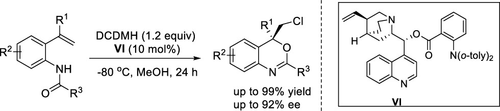
The catalytic asymmetric halocyclization of alkene is a powerful and straightforward strategy for the synthesis of chiral heterocyclic compounds. Herein, an effective approach to chiral benzoxazine derivatives through organocatalyzed chlorocyclization of o-vinylanilides was reported. This method provides facile access to a series of chiral benzoxazines in good to excellent yields (up to 99% yield) and with high-level enantiocontrol (up to 92% ee).
中文翻译:

通过邻乙烯基苯胺的催化不对称氯环化对映选择性合成4H-3,1-Benzoxazines。
烯烃的催化不对称卤代环化是合成手性杂环化合物的有力而直接的策略。在本文中,已经报道了通过邻乙烯基苯胺的有机催化的氯环化来制备手性苯并恶嗪衍生物的有效方法。该方法可以轻松获得一系列手性苯并恶嗪,收率好至极好(高达99%),并且具有高水平的对映体控制(ee高达92%)。
更新日期:2020-01-14
中文翻译:

通过邻乙烯基苯胺的催化不对称氯环化对映选择性合成4H-3,1-Benzoxazines。
烯烃的催化不对称卤代环化是合成手性杂环化合物的有力而直接的策略。在本文中,已经报道了通过邻乙烯基苯胺的有机催化的氯环化来制备手性苯并恶嗪衍生物的有效方法。该方法可以轻松获得一系列手性苯并恶嗪,收率好至极好(高达99%),并且具有高水平的对映体控制(ee高达92%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号