当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed Evolution Using Stabilized Bacterial Peptide Display
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/jacs.9b10716 Tejas Navaratna 1 , Lydia Atangcho 1 , Mukesh Mahajan 1 , Vivekanandan Subramanian 2 , Marshall Case 1 , Andrew Min 1 , Daniel Tresnak 1 , Greg M Thurber 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-12-27 , DOI: 10.1021/jacs.9b10716 Tejas Navaratna 1 , Lydia Atangcho 1 , Mukesh Mahajan 1 , Vivekanandan Subramanian 2 , Marshall Case 1 , Andrew Min 1 , Daniel Tresnak 1 , Greg M Thurber 1, 3
Affiliation
|
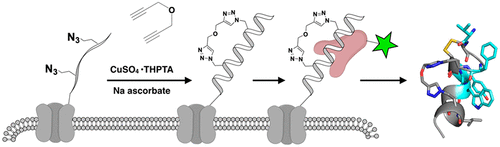
Chemically stabilized peptides have attracted intense interest by academics and pharmaceutical companies due to their potential to hit currently 'undruggable' targets. However, engineering an optimal sequence, stabilizing linker location, and physicochemical properties is a slow and arduous process. By pairing non-natural amino acid incorporation and cell surface click chemistry in bacteria with high-throughput sorting, we developed a method to quantitatively select high affinity ligands and applied the SPEED (Stabilized Peptide Evolution by E. coli Display) technique to develop disrupters of the therapeutically relevant MDM2-p53 interface. Through in situ stabilization on the bacterial surface, we demonstrate rapid isolation of stabilized peptides with improved affinity and novel structures. Several peptides evolved a second loop including one sequence (Kd = 1.8 nM) containing an i, i+4 disulfide bond. NMR structural determination indicated a bent helix in solution and bound to MDM2. The bicyclic peptide had improved protease stability, and we demonstrated that protease resistance could be measured both on the bacterial surface and in solution, enabling the method to test and/or screen for additional drug-like properties critical for biologically active compounds.
中文翻译:

使用稳定细菌肽展示的定向进化
化学稳定肽由于具有达到目前“不可成药”目标的潜力而引起了学术界和制药公司的浓厚兴趣。然而,设计最佳序列、稳定接头位置和物理化学特性是一个缓慢而艰巨的过程。通过将细菌中的非天然氨基酸掺入和细胞表面点击化学与高通量分选配对,我们开发了一种定量选择高亲和力配体的方法,并应用 SPEED(大肠杆菌展示稳定肽进化)技术开发治疗相关的 MDM2-p53 界面。通过在细菌表面原位稳定,我们证明了稳定肽的快速分离,具有更高的亲和力和新结构。几个肽进化出第二个环,包括一个含有 i, i+4 二硫键的序列 (Kd = 1.8 nM)。NMR 结构测定表明溶液中存在弯曲螺旋并与 MDM2 结合。双环肽具有改善的蛋白酶稳定性,我们证明可以在细菌表面和溶液中测量蛋白酶抗性,使该方法能够测试和/或筛选对生物活性化合物至关重要的其他类药物特性。
更新日期:2019-12-27
中文翻译:

使用稳定细菌肽展示的定向进化
化学稳定肽由于具有达到目前“不可成药”目标的潜力而引起了学术界和制药公司的浓厚兴趣。然而,设计最佳序列、稳定接头位置和物理化学特性是一个缓慢而艰巨的过程。通过将细菌中的非天然氨基酸掺入和细胞表面点击化学与高通量分选配对,我们开发了一种定量选择高亲和力配体的方法,并应用 SPEED(大肠杆菌展示稳定肽进化)技术开发治疗相关的 MDM2-p53 界面。通过在细菌表面原位稳定,我们证明了稳定肽的快速分离,具有更高的亲和力和新结构。几个肽进化出第二个环,包括一个含有 i, i+4 二硫键的序列 (Kd = 1.8 nM)。NMR 结构测定表明溶液中存在弯曲螺旋并与 MDM2 结合。双环肽具有改善的蛋白酶稳定性,我们证明可以在细菌表面和溶液中测量蛋白酶抗性,使该方法能够测试和/或筛选对生物活性化合物至关重要的其他类药物特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号