当前位置:
X-MOL 学术
›
PLOS Negl. Trop. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Treatment responses to Azithromycin and Ciprofloxacin in uncomplicated Salmonella Typhi infection: A comparison of Clinical and Microbiological Data from a Controlled Human Infection Model.
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2019-12-26 , DOI: 10.1371/journal.pntd.0007955 Celina Jin 1 , Malick M Gibani 1, 2 , Shaun H Pennington 3 , Xinxue Liu 1 , Alison Ardrey 3 , Ghaith Aljayyoussi 3 , Maria Moore 1 , Brian Angus 4 , Christopher M Parry 3, 5, 6, 7 , Giancarlo A Biagini 3 , Nicholas A Feasey 6, 8 , Andrew J Pollard 1
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2019-12-26 , DOI: 10.1371/journal.pntd.0007955 Celina Jin 1 , Malick M Gibani 1, 2 , Shaun H Pennington 3 , Xinxue Liu 1 , Alison Ardrey 3 , Ghaith Aljayyoussi 3 , Maria Moore 1 , Brian Angus 4 , Christopher M Parry 3, 5, 6, 7 , Giancarlo A Biagini 3 , Nicholas A Feasey 6, 8 , Andrew J Pollard 1
Affiliation
|
BACKGROUND
The treatment of enteric fever is complicated by the emergence of antimicrobial resistant Salmonella Typhi. Azithromycin is commonly used for first-line treatment of uncomplicated enteric fever, but the response to treatment may be sub-optimal in some patient groups when compared with fluoroquinolones.
METHODS
We performed an analysis of responses to treatment with azithromycin (500mg once-daily, 14 days) or ciprofloxacin (500mg twice-daily, 14 days) in healthy UK volunteers (18-60 years) enrolled into two Salmonella controlled human infection studies. Study A was a single-centre, open-label, randomised trial. Participants were randomised 1:1 to receive open-label oral ciprofloxacin or azithromycin, stratified by vaccine group (Vi-polysaccharide, Vi-conjugate or control Men-ACWY vaccine). Study B was an observational challenge/re-challenge study, where participants were randomised to challenge with Salmonella Typhi or Salmonella Paratyphi A. Outcome measures included fever clearance time, blood-culture clearance time and a composite measure of prolonged treatment response (persistent fever ≥38.0°C for ≥72 hours, persistently positive S. Typhi blood cultures for ≥72 hours, or change in antibiotic treatment). Both trials are registered with ClinicalTrials.gov (NCT02324751 and NCT02192008).
FINDINGS
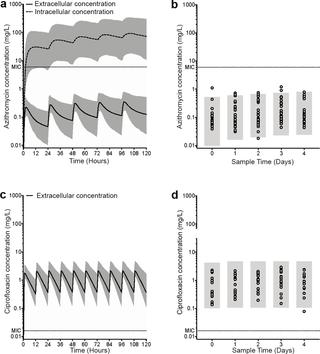
In 81 participants diagnosed with S. Typhi in two studies, treatment with azithromycin was associated with prolonged bacteraemia (median 90.8 hours [95% CI: 65.9-93.8] vs. 20.1 hours [95% CI: 7.8-24.3], p<0.001) and prolonged fever clearance times <37.5°C (hazard ratio 2.4 [95%CI: 1.2-5.0]; p = 0.02). Results were consistent when studies were analysed independently and in a sub-group of participants with no history of vaccination or previous challenge. A prolonged treatment response was observed significantly more frequently in the azithromycin group (28/52 [54.9%]) compared with the ciprofloxacin group (1/29 [3.5%]; p<0.001). In participants treated with azithromycin, observed systemic plasma concentrations of azithromycin did not exceed the minimum inhibitory concentration (MIC), whilst predicted intracellular concentrations did exceed the MIC. In participants treated with ciprofloxacin, the observed systemic plasma concentrations and predicted intracellular concentrations of ciprofloxacin exceeded the MIC.
INTERPRETATION
Azithromycin at a dose of 500mg daily is an effective treatment for fully sensitive strains of S. Typhi but is associated with delayed treatment response and prolonged bacteraemia when compared with ciprofloxacin within the context of a human challenge model. Whilst the cellular accumulation of azithromycin is predicted to be sufficient to treat intracellular S. Typhi, systemic exposure may be sub-optimal for the elimination of extracellular circulating S. Typhi. In an era of increasing antimicrobial resistance, further studies are required to define appropriate azithromycin dosing regimens for enteric fever and to assess novel treatment strategies, including combination therapies.
TRIAL REGISTRATION
ClinicalTrials.gov (NCT02324751 and NCT02192008).
中文翻译:

无并发症伤寒沙门氏菌感染对阿奇霉素和环丙沙星的治疗反应:对照人类感染模型的临床和微生物学数据的比较。
背景技术肠热病的治疗因伤寒沙门氏菌耐药性的出现而变得复杂。阿奇霉素通常用于无并发症肠热的一线治疗,但与氟喹诺酮类药物相比,某些患者组的治疗反应可能不是最佳。方法 我们对参加两项沙门氏菌对照人类感染研究的健康英国志愿者(18-60 岁)对阿奇霉素(500 毫克,每日一次,14 天)或环丙沙星(500 毫克,每日两次,14 天)治疗的反应进行了分析。研究 A 是一项单中心、开放标签、随机试验。参与者以 1:1 的比例随机接受开放标签口服环丙沙星或阿奇霉素,并按疫苗组(Vi-多糖、Vi-结合物或对照 Men-ACWY 疫苗)分层。研究 B 是一项观察性攻击/再攻击研究,参与者被随机分配接受伤寒沙门氏菌或甲型副伤寒沙门氏菌攻击。结果测量包括发烧清除时间、血培养清除时间和延长治疗反应的综合测量(持续发烧≥ 38.0°C ≥72 小时,伤寒沙门氏菌血培养持续阳性≥72 小时,或改变抗生素治疗)。这两项试验均已在 ClinicalTrials.gov 注册(NCT02324751 和 NCT02192008)。结果 在两项研究中,在 81 名被诊断患有伤寒沙门氏菌的参与者中,阿奇霉素治疗与长期菌血症相关(中位时间 90.8 小时 [95% CI: 65.9-93.8] 对比 20.1 小时 [95% CI: 7.8-24.3],p< 0.001)和延长退烧时间 <37.5°C(风险比 2.4 [95%CI:1.2-5.0];p = 0.02)。当对没有疫苗接种史或既往挑战史的参与者亚组进行独立分析时,结果是一致的。 与环丙沙星组 (1/29 [3.5%];p<0.001) 相比,阿奇霉素组 (28/52 [54.9%]) 观察到治疗反应延长的频率明显更高。在接受阿奇霉素治疗的参与者中,观察到的阿奇霉素全身血浆浓度没有超过最低抑制浓度 (MIC),而预测的细胞内浓度确实超过了 MIC。在接受环丙沙星治疗的参与者中,观察到的环丙沙星全身血浆浓度和预测的环丙沙星细胞内浓度超过了 MIC。解释 每日 500mg 剂量的阿奇霉素可有效治疗完全敏感的伤寒沙门氏菌菌株,但在人类攻击模型中与环丙沙星相比,阿奇霉素会导致治疗反应延迟和菌血症延长。虽然阿奇霉素的细胞积聚预计足以治疗细胞内伤寒沙门氏菌,但全身暴露对于消除细胞外循环伤寒沙门氏菌可能不是最佳选择。在抗菌药物耐药性日益增加的时代,需要进一步研究来确定肠热病适当的阿奇霉素给药方案,并评估新的治疗策略,包括联合疗法。试验注册 ClinicalTrials.gov(NCT02324751 和 NCT02192008)。
更新日期:2019-12-27
中文翻译:

无并发症伤寒沙门氏菌感染对阿奇霉素和环丙沙星的治疗反应:对照人类感染模型的临床和微生物学数据的比较。
背景技术肠热病的治疗因伤寒沙门氏菌耐药性的出现而变得复杂。阿奇霉素通常用于无并发症肠热的一线治疗,但与氟喹诺酮类药物相比,某些患者组的治疗反应可能不是最佳。方法 我们对参加两项沙门氏菌对照人类感染研究的健康英国志愿者(18-60 岁)对阿奇霉素(500 毫克,每日一次,14 天)或环丙沙星(500 毫克,每日两次,14 天)治疗的反应进行了分析。研究 A 是一项单中心、开放标签、随机试验。参与者以 1:1 的比例随机接受开放标签口服环丙沙星或阿奇霉素,并按疫苗组(Vi-多糖、Vi-结合物或对照 Men-ACWY 疫苗)分层。研究 B 是一项观察性攻击/再攻击研究,参与者被随机分配接受伤寒沙门氏菌或甲型副伤寒沙门氏菌攻击。结果测量包括发烧清除时间、血培养清除时间和延长治疗反应的综合测量(持续发烧≥ 38.0°C ≥72 小时,伤寒沙门氏菌血培养持续阳性≥72 小时,或改变抗生素治疗)。这两项试验均已在 ClinicalTrials.gov 注册(NCT02324751 和 NCT02192008)。结果 在两项研究中,在 81 名被诊断患有伤寒沙门氏菌的参与者中,阿奇霉素治疗与长期菌血症相关(中位时间 90.8 小时 [95% CI: 65.9-93.8] 对比 20.1 小时 [95% CI: 7.8-24.3],p< 0.001)和延长退烧时间 <37.5°C(风险比 2.4 [95%CI:1.2-5.0];p = 0.02)。当对没有疫苗接种史或既往挑战史的参与者亚组进行独立分析时,结果是一致的。 与环丙沙星组 (1/29 [3.5%];p<0.001) 相比,阿奇霉素组 (28/52 [54.9%]) 观察到治疗反应延长的频率明显更高。在接受阿奇霉素治疗的参与者中,观察到的阿奇霉素全身血浆浓度没有超过最低抑制浓度 (MIC),而预测的细胞内浓度确实超过了 MIC。在接受环丙沙星治疗的参与者中,观察到的环丙沙星全身血浆浓度和预测的环丙沙星细胞内浓度超过了 MIC。解释 每日 500mg 剂量的阿奇霉素可有效治疗完全敏感的伤寒沙门氏菌菌株,但在人类攻击模型中与环丙沙星相比,阿奇霉素会导致治疗反应延迟和菌血症延长。虽然阿奇霉素的细胞积聚预计足以治疗细胞内伤寒沙门氏菌,但全身暴露对于消除细胞外循环伤寒沙门氏菌可能不是最佳选择。在抗菌药物耐药性日益增加的时代,需要进一步研究来确定肠热病适当的阿奇霉素给药方案,并评估新的治疗策略,包括联合疗法。试验注册 ClinicalTrials.gov(NCT02324751 和 NCT02192008)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号