当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of binding residues between periplasmic adapter protein (PAP) and RND efflux pumps explains PAP-pump promiscuity and roles in antimicrobial resistance.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2019-12-26 , DOI: 10.1371/journal.ppat.1008101 Helen E McNeil 1 , Ilyas Alav 1 , Ricardo Corona Torres 2 , Amanda E Rossiter 1 , Eve Laycock 1 , Simon Legood 1 , Inderpreet Kaur 1 , Matthew Davies 1 , Matthew Wand 3 , Mark A Webber 4 , Vassiliy N Bavro 2 , Jessica M A Blair 1
PLoS Pathogens ( IF 5.5 ) Pub Date : 2019-12-26 , DOI: 10.1371/journal.ppat.1008101 Helen E McNeil 1 , Ilyas Alav 1 , Ricardo Corona Torres 2 , Amanda E Rossiter 1 , Eve Laycock 1 , Simon Legood 1 , Inderpreet Kaur 1 , Matthew Davies 1 , Matthew Wand 3 , Mark A Webber 4 , Vassiliy N Bavro 2 , Jessica M A Blair 1
Affiliation
|
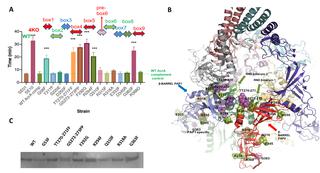
Active efflux due to tripartite RND efflux pumps is an important mechanism of clinically relevant antibiotic resistance in Gram-negative bacteria. These pumps are also essential for Gram-negative pathogens to cause infection and form biofilms. They consist of an inner membrane RND transporter; a periplasmic adaptor protein (PAP), and an outer membrane channel. The role of PAPs in assembly, and the identities of specific residues involved in PAP-RND binding, remain poorly understood. Using recent high-resolution structures, four 3D sites involved in PAP-RND binding within each PAP protomer were defined that correspond to nine discrete linear binding sequences or "binding boxes" within the PAP sequence. In the important human pathogen Salmonella enterica, these binding boxes are conserved within phylogenetically-related PAPs, such as AcrA and AcrE, while differing considerably between divergent PAPs such as MdsA and MdtA, despite overall conservation of the PAP structure. By analysing these binding sequences we created a predictive model of PAP-RND interaction, which suggested the determinants that may allow promiscuity between certain PAPs, but discrimination of others. We corroborated these predictions using direct phenotypic data, confirming that only AcrA and AcrE, but not MdtA or MsdA, can function with the major RND pump AcrB. Furthermore, we provide functional validation of the involvement of the binding boxes by disruptive site-directed mutagenesis. These results directly link sequence conservation within identified PAP binding sites with functional data providing mechanistic explanation for assembly of clinically relevant RND-pumps and explain how Salmonella and other pathogens maintain a degree of redundancy in efflux mediated resistance. Overall, our study provides a novel understanding of the molecular determinants driving the RND-PAP recognition by bridging the available structural information with experimental functional validation thus providing the scientific community with a predictive model of pump-contacts that could be exploited in the future for the development of targeted therapeutics and efflux pump inhibitors.
中文翻译:

鉴定周质衔接蛋白(PAP)和RND外排泵之间的结合残基可以解释PAP泵的混杂性及其在抗菌素耐药性中的作用。
三方RND外排泵引起的主动外排是革兰氏阴性细菌临床相关抗生素耐药性的重要机制。这些泵对于革兰氏阴性病原体引起感染并形成生物膜也是必不可少的。它们由内膜RND转运蛋白组成;周质衔接蛋白(PAP)和外膜通道。PAP在组装中的作用以及与PAP-RND结合所涉及的特定残基的身份仍然知之甚少。使用最新的高分辨率结构,定义了每个PAP前体内参与PAP-RND结合的四个3D位置,它们对应于PAP序列内的九个离散线性结合序列或“结合盒”。在重要的人类病原体小肠沙门氏菌中,这些结合盒在系统发育相关的PAP中是保守的,尽管APA的总体结构保持不变,但MPA和MDTA等不同的PAP之间的区别却很大。通过分析这些结合序列,我们创建了PAP-RND相互作用的预测模型,该模型提出了可能允许某些PAP之间混杂但可区分其他PAP的决定因素。我们使用直接的表型数据证实了这些预测,确认只有AcrA和AcrE,而MdtA或MsdA不能与主要RND泵AcrB一起使用。此外,我们通过破坏性定点诱变提供了对结合盒参与的功能验证。这些结果直接将鉴定的PAP结合位点内的序列保守性与功能数据联系起来,为组装临床相关的RND泵提供了机械解释,并解释了沙门氏菌和其他病原体如何在外排介导的抗性中保持一定程度的冗余。总体而言,我们的研究通过将可用的结构信息与实验功能验证结合在一起,从而对驱动RND-PAP识别的分子决定因素提供了新颖的理解,从而为科学界提供了泵接触的预测模型,该模型可在将来用于靶向治疗药物和外排泵抑制剂的开发。
更新日期:2019-12-27
中文翻译:

鉴定周质衔接蛋白(PAP)和RND外排泵之间的结合残基可以解释PAP泵的混杂性及其在抗菌素耐药性中的作用。
三方RND外排泵引起的主动外排是革兰氏阴性细菌临床相关抗生素耐药性的重要机制。这些泵对于革兰氏阴性病原体引起感染并形成生物膜也是必不可少的。它们由内膜RND转运蛋白组成;周质衔接蛋白(PAP)和外膜通道。PAP在组装中的作用以及与PAP-RND结合所涉及的特定残基的身份仍然知之甚少。使用最新的高分辨率结构,定义了每个PAP前体内参与PAP-RND结合的四个3D位置,它们对应于PAP序列内的九个离散线性结合序列或“结合盒”。在重要的人类病原体小肠沙门氏菌中,这些结合盒在系统发育相关的PAP中是保守的,尽管APA的总体结构保持不变,但MPA和MDTA等不同的PAP之间的区别却很大。通过分析这些结合序列,我们创建了PAP-RND相互作用的预测模型,该模型提出了可能允许某些PAP之间混杂但可区分其他PAP的决定因素。我们使用直接的表型数据证实了这些预测,确认只有AcrA和AcrE,而MdtA或MsdA不能与主要RND泵AcrB一起使用。此外,我们通过破坏性定点诱变提供了对结合盒参与的功能验证。这些结果直接将鉴定的PAP结合位点内的序列保守性与功能数据联系起来,为组装临床相关的RND泵提供了机械解释,并解释了沙门氏菌和其他病原体如何在外排介导的抗性中保持一定程度的冗余。总体而言,我们的研究通过将可用的结构信息与实验功能验证结合在一起,从而对驱动RND-PAP识别的分子决定因素提供了新颖的理解,从而为科学界提供了泵接触的预测模型,该模型可在将来用于靶向治疗药物和外排泵抑制剂的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号