Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Na+ A1 AO ATP synthase with a V-type c subunit in a mesophilic bacterium.
The FEBS Journal ( IF 5.4 ) Pub Date : 2019-12-26 , DOI: 10.1111/febs.15193 Dennis Litty 1 , Volker Müller 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2019-12-26 , DOI: 10.1111/febs.15193 Dennis Litty 1 , Volker Müller 1
Affiliation
|
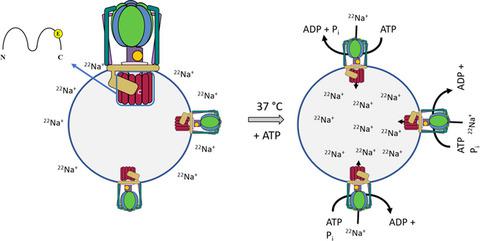
A1AO ATP synthases with a V‐type c subunit have only been found in hyperthermophilic archaea which makes bioenergetic analyses impossible due to the instability of liposomes at high temperatures. A search for a potential archaeal A1AO ATP synthase with a V‐type c subunit in a mesophilic organism revealed an A1AO ATP synthase cluster in the anaerobic, acetogenic bacterium Eubacterium limosum KIST612. The enzyme was purified to apparent homogeneity from cells grown on methanol to a specific activity of 1.2 U·mg−1 with a yield of 12%. The enzyme contained subunits A, B, C, D, E, F, H, a , and c . Subunit c is predicted to be a typical V‐type c subunit with only one ion (Na+)‐binding site. Indeed, ATP hydrolysis was strictly Na+‐dependent. N ,N ′‐dicyclohexylcarbodiimide (DCCD) inhibited ATP hydrolysis, but inhibition was relieved by addition of Na+. Na+ was shown directly to abolish binding of the fluorescence DCCD derivative, NCD‐4, to subunit c , demonstrating a competition of Na+ and DCCD/NCD‐4 for a common binding site. After incorporation of the A1AO ATP synthase into liposomes, ATP‐dependent primary transport of 22Na+ as well as ΔµNa+‐driven ATP synthesis could be demonstrated. The Na+ A1AO ATP synthase from E. limosum is the first ATP synthase with a V‐type c subunit from a mesophilic organism. This will enable future bioenergetic analysis of these unique ATP synthases.
中文翻译:

在嗜温细菌中具有V型c亚基的Na + A1 AO ATP合酶。
仅在超嗜热古细菌中发现具有V型c亚基的1 A O ATP合成酶,由于脂质体在高温下不稳定,因此无法进行生物能分析。一种用于潜在古细菌甲搜索1甲ö ATP与V型合酶Ç在嗜温生物亚基揭示的A 1甲ö ATP合酶集群中的厌氧的,产乙酸细菌真杆菌limosum KIST612。从在甲醇中生长至比活度为1.2 U·mg -1的细胞中纯化出的酶具有明显的均质性收率为12%。该酶包含亚基A,B,C,D,E,F,H,a和c。c亚基被预测为典型的V型c亚基,只有一个离子(Na +)结合位点。确实,ATP水解严格依赖于Na +依赖。N,N'-二环己基碳二亚胺(DCCD)抑制ATP水解,但通过添加Na +解除了抑制作用。直接显示Na +消除了荧光DCCD衍生物NCD-4与c亚基的结合,表明Na +的竞争和DCCD / NCD-4(用于共同的结合位点)。将A 1 A O ATP合酶掺入脂质体后,可以证明ATP依赖的22 Na +初级转运以及ΔµNa +驱动的ATP合成。源自E. limosum的Na + A 1 A O ATP合酶是嗜温生物中具有V型c亚基的第一个ATP合酶。这将使将来能够对这些独特的ATP合酶进行生物能分析。
更新日期:2019-12-26
中文翻译:

在嗜温细菌中具有V型c亚基的Na + A1 AO ATP合酶。
仅在超嗜热古细菌中发现具有V型c亚基的1 A O ATP合成酶,由于脂质体在高温下不稳定,因此无法进行生物能分析。一种用于潜在古细菌甲搜索1甲ö ATP与V型合酶Ç在嗜温生物亚基揭示的A 1甲ö ATP合酶集群中的厌氧的,产乙酸细菌真杆菌limosum KIST612。从在甲醇中生长至比活度为1.2 U·mg -1的细胞中纯化出的酶具有明显的均质性收率为12%。该酶包含亚基A,B,C,D,E,F,H,a和c。c亚基被预测为典型的V型c亚基,只有一个离子(Na +)结合位点。确实,ATP水解严格依赖于Na +依赖。N,N'-二环己基碳二亚胺(DCCD)抑制ATP水解,但通过添加Na +解除了抑制作用。直接显示Na +消除了荧光DCCD衍生物NCD-4与c亚基的结合,表明Na +的竞争和DCCD / NCD-4(用于共同的结合位点)。将A 1 A O ATP合酶掺入脂质体后,可以证明ATP依赖的22 Na +初级转运以及ΔµNa +驱动的ATP合成。源自E. limosum的Na + A 1 A O ATP合酶是嗜温生物中具有V型c亚基的第一个ATP合酶。这将使将来能够对这些独特的ATP合酶进行生物能分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号