Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Human KCNQ1 Modulation and Gating.
Cell ( IF 45.5 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.cell.2019.12.003 Ji Sun 1 , Roderick MacKinnon 1
Cell ( IF 45.5 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.cell.2019.12.003 Ji Sun 1 , Roderick MacKinnon 1
Affiliation
|
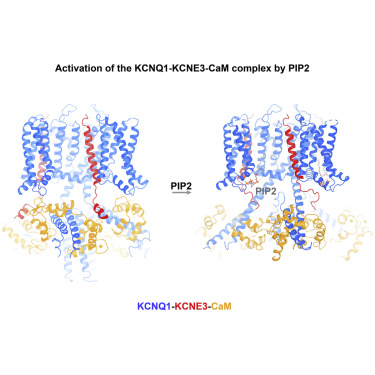
KCNQ1, also known as Kv7.1, is a voltage-dependent K+ channel that regulates gastric acid secretion, salt and glucose homeostasis, and heart rhythm. Its functional properties are regulated in a tissue-specific manner through co-assembly with beta subunits KCNE1-5. In non-excitable cells, KCNQ1 forms a complex with KCNE3, which suppresses channel closure at negative membrane voltages that otherwise would close it. Pore opening is regulated by the signaling lipid PIP2. Using cryoelectron microscopy (cryo-EM), we show that KCNE3 tucks its single-membrane-spanning helix against KCNQ1, at a location that appears to lock the voltage sensor in its depolarized conformation. Without PIP2, the pore remains closed. Upon addition, PIP2 occupies a site on KCNQ1 within the inner membrane leaflet, which triggers a large conformational change that leads to dilation of the pore's gate. It is likely that this mechanism of PIP2 activation is conserved among Kv7 channels.
中文翻译:

人类 KCNQ1 调制和门控的结构基础。
KCNQ1,也称为 Kv7.1,是一种电压依赖性 K+ 通道,可调节胃酸分泌、盐和葡萄糖稳态以及心律。它的功能特性通过与β亚基KCNE1-5的共组装以组织特异性方式进行调节。在不可兴奋细胞中,KCNQ1 与 KCNE3 形成复合物,在负膜电压下抑制通道关闭,否则会关闭通道。孔开口由信号脂质 PIP2 调节。使用低温电子显微镜 (cryo-EM),我们发现 KCNE3 将其单膜跨螺旋线折叠在 KCNQ1 上,该位置似乎将电压传感器锁定在其去极化构象中。如果没有 PIP2,孔将保持关闭状态。添加后,PIP2 在内膜小叶内占据 KCNQ1 上的一个位点,这会触发大的构象变化,从而导致孔门的扩张。这种 PIP2 激活机制很可能在 Kv7 通道中是保守的。
更新日期:2019-12-27
中文翻译:

人类 KCNQ1 调制和门控的结构基础。
KCNQ1,也称为 Kv7.1,是一种电压依赖性 K+ 通道,可调节胃酸分泌、盐和葡萄糖稳态以及心律。它的功能特性通过与β亚基KCNE1-5的共组装以组织特异性方式进行调节。在不可兴奋细胞中,KCNQ1 与 KCNE3 形成复合物,在负膜电压下抑制通道关闭,否则会关闭通道。孔开口由信号脂质 PIP2 调节。使用低温电子显微镜 (cryo-EM),我们发现 KCNE3 将其单膜跨螺旋线折叠在 KCNQ1 上,该位置似乎将电压传感器锁定在其去极化构象中。如果没有 PIP2,孔将保持关闭状态。添加后,PIP2 在内膜小叶内占据 KCNQ1 上的一个位点,这会触发大的构象变化,从而导致孔门的扩张。这种 PIP2 激活机制很可能在 Kv7 通道中是保守的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号