当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of novel indole-pyrazoline hybrid derivatives as potential topoisomerase 1 inhibitors.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.bmcl.2019.126925 Bing Shu 1 , Qian Yu 2 , De-Xuan Hu 3 , Tong Che 4 , Shang-Shi Zhang 4 , Ding Li 3
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.bmcl.2019.126925 Bing Shu 1 , Qian Yu 2 , De-Xuan Hu 3 , Tong Che 4 , Shang-Shi Zhang 4 , Ding Li 3
Affiliation
|
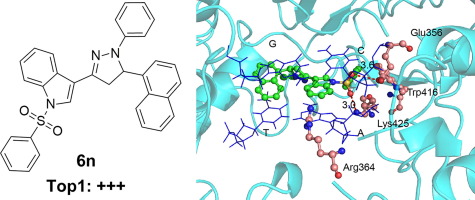
A series of novel indole-pyrazoline hybrid derivatives were designed, synthesized, and evaluated for topoisomerase 1 (Top1) inhibitory activity. Top1-mediated relaxation assays showed that our synthesized compounds had variable Top1 inhibitory activity. Among these compounds, 3-(5-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl)-1-(phenylsulfonyl)-1H-indole (6n) was found to be a strong Top1 inhibitor with better inhibitory activity than CPT and hit compounds. Our further experiments rationalized the mode of action for this new type of inhibitors, which showed no significant binding to supercoiled DNA.
中文翻译:

作为潜在的拓扑异构酶1抑制剂的新型吲哚-吡唑啉杂化衍生物的合成和生物学评估。
设计,合成和评估了一系列新型的吲哚-吡唑啉杂合衍生物的拓扑异构酶1(Top1)抑制活性。Top1介导的弛豫分析表明,我们合成的化合物具有可变的Top1抑制活性。在这些化合物中,发现了3-(5-(萘-1-基)-1-苯基-4,5-二氢-1H-吡唑-3-基)-1-(苯磺酰基)-1H-吲哚(6n)成为强力的Top1抑制剂,具有比CPT和命中化合物更好的抑制活性。我们的进一步实验合理化了这种新型抑制剂的作用方式,该抑制剂未显示与超螺旋DNA的显着结合。
更新日期:2019-12-27
中文翻译:

作为潜在的拓扑异构酶1抑制剂的新型吲哚-吡唑啉杂化衍生物的合成和生物学评估。
设计,合成和评估了一系列新型的吲哚-吡唑啉杂合衍生物的拓扑异构酶1(Top1)抑制活性。Top1介导的弛豫分析表明,我们合成的化合物具有可变的Top1抑制活性。在这些化合物中,发现了3-(5-(萘-1-基)-1-苯基-4,5-二氢-1H-吡唑-3-基)-1-(苯磺酰基)-1H-吲哚(6n)成为强力的Top1抑制剂,具有比CPT和命中化合物更好的抑制活性。我们的进一步实验合理化了这种新型抑制剂的作用方式,该抑制剂未显示与超螺旋DNA的显着结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号