当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convenient synthesis of novel sulfonamide derivatives as promising anticancer agents
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-26 , DOI: 10.1002/jhet.3849 Ahmed El‐Mekabaty 1 , Hanem M. Awad 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-26 , DOI: 10.1002/jhet.3849 Ahmed El‐Mekabaty 1 , Hanem M. Awad 2
Affiliation
|
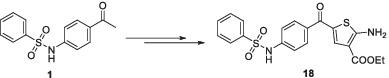
Novel sulfonamide derivatives have been synthesized from the readily accessible N‐(4‐acetylphenyl)benzenesulfonamide (1). Condensation of 1 with phenylhydrazine in refluxing ethyl alcohol gave the corresponding phenylhydrazone 2, which was then added to the Vilsmeier‐Haack reagent (POCl3/DMF) to give the 4‐formylpyrazole derivative 3. Fusion of 1 with thiourea in the presence of iodine at 130°C afforded the 2‐aminothiazole derivative 4. Refluxing 1 with an excess of N, N‐dimethylformamide dimethyl acetal furnished the enaminone 5. The chemical reactivity of enaminone 5 toward some nitrogen and carbon nucleophiles has been studied to obtain polyfunctionalized heteroaromatic systems bearing a sulfonamide moiety. Besides, the enaminone 5 undergoes the Gewald reaction and reacts with ethyl cyanoacetate and elemental sulfur in the presence of morpholine to yield the 2‐aminothiophene derivative 18. Moreover, the utility of 5 for the synthesis of 4‐(phenylsulfonamido)benzoic acid (19) was investigated. The synthesized sulfonamides were evaluated for their in vitro cytotoxic activities against two human cell lines, MCF‐7 (breast adenocarcinoma cells) and RPE‐1 (normal retina pigmented epithelium cells). The results revealed that compounds 1‐3, 6‐8, 10, 12b, 18, 19, and 21 have a potent cytotoxic effect on MCF‐7 and less on RPE‐1 cells compared to the positive control doxorubicin®.
中文翻译:

方便合成新型磺酰胺衍生物作为有前途的抗癌药
新型磺酰胺衍生物是由易于获得的N-(4-乙酰基苯基)苯磺酰胺合成的(1)。1与苯肼在回流的乙醇中缩合,得到相应的苯hydr 2,然后将其加入Vilsmeier-Haack试剂(POCl 3 / DMF)中,得到4-甲酰基吡唑衍生物3。在碘存在下于130°C将1与硫脲融合,得到2-氨基噻唑衍生物4。用过量的N,N-二甲基甲酰胺二甲基乙缩醛回流1提供了烯胺酮5。已经研究了烯胺5对某些氮和碳亲核试剂的化学反应性,以获得带有磺酰胺部分的多官能杂芳族体系。此外,烯胺5发生Gewald反应,并在吗啉存在下与氰基乙酸乙酯和元素硫反应,生成2-氨基噻吩衍生物18。此外,还研究了5在合成4-(苯磺酰胺基)苯甲酸(19)中的效用。对合成的磺酰胺类药物进行了体外评估对两种人类细胞系MCF-7(乳腺癌细胞)和RPE-1(正常的视网膜色素上皮细胞)具有细胞毒活性。结果表明,化合物1-3,6-8,10,12B,18,19,和21对MCF-7的强效的细胞毒性作用而减少对RPE-1细胞相比,阳性对照doxorubicin®。
更新日期:2019-12-27
中文翻译:

方便合成新型磺酰胺衍生物作为有前途的抗癌药
新型磺酰胺衍生物是由易于获得的N-(4-乙酰基苯基)苯磺酰胺合成的(1)。1与苯肼在回流的乙醇中缩合,得到相应的苯hydr 2,然后将其加入Vilsmeier-Haack试剂(POCl 3 / DMF)中,得到4-甲酰基吡唑衍生物3。在碘存在下于130°C将1与硫脲融合,得到2-氨基噻唑衍生物4。用过量的N,N-二甲基甲酰胺二甲基乙缩醛回流1提供了烯胺酮5。已经研究了烯胺5对某些氮和碳亲核试剂的化学反应性,以获得带有磺酰胺部分的多官能杂芳族体系。此外,烯胺5发生Gewald反应,并在吗啉存在下与氰基乙酸乙酯和元素硫反应,生成2-氨基噻吩衍生物18。此外,还研究了5在合成4-(苯磺酰胺基)苯甲酸(19)中的效用。对合成的磺酰胺类药物进行了体外评估对两种人类细胞系MCF-7(乳腺癌细胞)和RPE-1(正常的视网膜色素上皮细胞)具有细胞毒活性。结果表明,化合物1-3,6-8,10,12B,18,19,和21对MCF-7的强效的细胞毒性作用而减少对RPE-1细胞相比,阳性对照doxorubicin®。











































 京公网安备 11010802027423号
京公网安备 11010802027423号