当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Chiral β‐Lactams by Pd‐Catalyzed Enantioselective Amidation of Methylene C(sp3)–H Bonds
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-01-10 , DOI: 10.1002/cjoc.201900533 Tao Zhou 1 , Meng‐Xue Jiang 2, 3 , Xu Yang 2, 3 , Qiang Yue 1 , Ye‐Qiang Han 1 , Yi Ding 1 , Bing‐Feng Shi 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-01-10 , DOI: 10.1002/cjoc.201900533 Tao Zhou 1 , Meng‐Xue Jiang 2, 3 , Xu Yang 2, 3 , Qiang Yue 1 , Ye‐Qiang Han 1 , Yi Ding 1 , Bing‐Feng Shi 1
Affiliation
|
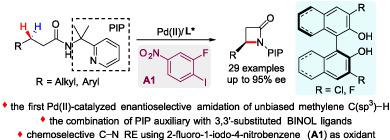
A Pd(II)‐catalyzed enantioselective intramolecular amidation of both benzylic and unbiased methylene C(sp3)−H bonds for the straightforward synthesis of chiral β‐lactams from aliphatic carboxamides is reported. The combination of 2‐pyridinylisopropyl (PIP) auxiliary with 3,3’‐substituted BINOL ligands is crucial for the enhancement of both reactivity and enantiocontrol of differentiating unbiased methylene C(sp3)−H bonds. The desired chemoselective C—N reductive elimination was achieved by employing 2‐fluoro‐1‐iodo‐4‐nitrobenzene as oxidant.
中文翻译:

钯催化亚甲基C(sp3)-H键对映选择性酰胺化反应合成手性β-内酰胺
有报道称,Pd(II)催化的苄基和无偏亚甲基C(sp 3)-H键均由对映选择性分子内酰胺化,可直接由脂肪族羧酰胺合成手性β-内酰胺。2-吡啶基异丙基(PIP)助剂与3,3'-取代的BINOL配体的结合对于增强反应性和对映性控制无偏亚甲基C(sp 3)-H键的区分至关重要。通过使用2-氟-1-碘-4-硝基苯作为氧化剂,可以实现所需的化学选择性CN还原消除。
更新日期:2020-01-11
中文翻译:

钯催化亚甲基C(sp3)-H键对映选择性酰胺化反应合成手性β-内酰胺
有报道称,Pd(II)催化的苄基和无偏亚甲基C(sp 3)-H键均由对映选择性分子内酰胺化,可直接由脂肪族羧酰胺合成手性β-内酰胺。2-吡啶基异丙基(PIP)助剂与3,3'-取代的BINOL配体的结合对于增强反应性和对映性控制无偏亚甲基C(sp 3)-H键的区分至关重要。通过使用2-氟-1-碘-4-硝基苯作为氧化剂,可以实现所需的化学选择性CN还原消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号