当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pentafluorophenyl Groups as Remote Substituents in Ni(II) Polymerization Catalysis
Organometallics ( IF 2.5 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.organomet.9b00784 Manuel Schnitte 1 , Sophia Lipinski 1 , Eva Schiebel 1 , Stefan Mecking 1
Organometallics ( IF 2.5 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.organomet.9b00784 Manuel Schnitte 1 , Sophia Lipinski 1 , Eva Schiebel 1 , Stefan Mecking 1
Affiliation
|
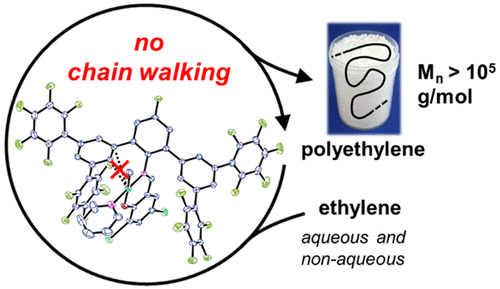
Perfluoroaromatic molecules have been studied intensively as versatile electron-poor π systems. We demonstrate the use of pentafluorophenyl substituents in remote positions of Ni(II) salicylaldiminato catalysts for ethylene polymerization. With their strongly electron-withdrawing character, high molecular weights of Mn > 105 g/mol and simultaneously low degrees of branching are accessible due to an efficient suppression of β-hydride elimination. This can be ascribed to suppressed nickel–aryl interactions and reduced electron densities at the metal center, as concluded from X-ray crystal structure analysis and electrochemical studies. The pentafluorophenyl-substituted catalyst is stable under aqueous conditions for direct polymerization to dispersions of well-defined nanocrystals.
中文翻译:

五氟苯基作为Ni(II)聚合催化中的远程取代基
全氟芳族分子已被广泛研究为通用的贫电子π系统。我们证明了在乙烯聚合的Ni(II)水杨基亚胺基化催化剂的偏远位置使用五氟苯基取代基。由于具有很强的吸电子特性,M n > 10 5的高分子量由于有效抑制了β-氢化物的消除,因此可以达到g / mol和低支化度。从X射线晶体结构分析和电化学研究得出的结论是,这可以归因于镍-芳基相互作用的抑制和金属中心电子密度的降低。五氟苯基取代的催化剂在水性条件下稳定,可直接聚合成明确定义的纳米晶体分散体。
更新日期:2019-12-27
中文翻译:

五氟苯基作为Ni(II)聚合催化中的远程取代基
全氟芳族分子已被广泛研究为通用的贫电子π系统。我们证明了在乙烯聚合的Ni(II)水杨基亚胺基化催化剂的偏远位置使用五氟苯基取代基。由于具有很强的吸电子特性,M n > 10 5的高分子量由于有效抑制了β-氢化物的消除,因此可以达到g / mol和低支化度。从X射线晶体结构分析和电化学研究得出的结论是,这可以归因于镍-芳基相互作用的抑制和金属中心电子密度的降低。五氟苯基取代的催化剂在水性条件下稳定,可直接聚合成明确定义的纳米晶体分散体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号