当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural preferences of cysteine sulfinic acid: The sulfinate engages in multiple local interactions with the peptide backbone.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.freeradbiomed.2019.12.030 Andrew R Urmey 1 , Neal J Zondlo 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.freeradbiomed.2019.12.030 Andrew R Urmey 1 , Neal J Zondlo 1
Affiliation
|
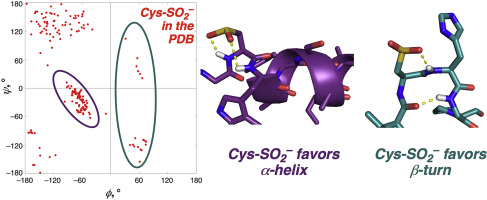
Cysteine sulfinic acid (Cys-SO2-) is a non-enzymatic oxidative post-translational modification (PTM) that has been identified in hundreds of proteins. However, the effects of cysteine sulfination are in most cases poorly understood. Cys-SO2- is structurally distinctive, with long sulfur-carbon and sulfur-oxygen bonds, and with tetrahedral geometry around sulfur due to its lone pair. Cys-SO2- thus has a unique range of potential interactions with the protein backbone which could facilitate protein structural changes. Herein, the structural effects of cysteine oxidation to the sulfinic acid were investigated in model peptides and folded proteins using NMR spectroscopy, circular dichroism, bioinformatics, and computational studies. In the PDB, Cys-SO2- shows a greater preference for α-helix than Cys. In addition, Cys-SO2- is more commonly found in structures with φ > 0, including in multiple types of β-turn. Sulfinate oxygens engage in hydrogen bonds with adjacent (i or i + 1) amide hydrogens. Over half of sulfinates have at least one hydrogen bond with an adjacent amide, and several structures have hydrogen bonds with both adjacent amides. Alternately, sulfur or either oxygen can act as an electron donor for n→π* interactions with the backbone carbonyl of the same residue, as indicated by frequent S⋯CO or O⋯CO distances below the sums of their van der Waals radii in protein structures. In peptides, Cys-SO2- favored α-helical structure at the N-terminus, consistent with helix dipole effects and backbone hydrogen bonds with the sulfinate promoting α-helix. Cys-SO2- has only modestly greater polyproline II helix propensity than Cys-SH, likely due to competition from multiple side chain-backbone interactions. Cys-SO2- stabilizes the i+1 position of a β-turn relative to Cys-SH. Within proteins, the range of side chain-main chain interactions available to Cys-SO2- compared to Cys-SH provides a basis for potential changes in protein structure and function due to cysteine oxidation to the sulfinic acid.
中文翻译:

半胱氨酸亚磺酸的结构偏好:亚磺酸盐与肽骨架进行多种局部相互作用。
半胱氨酸亚磺酸 (Cys-SO2-) 是一种非酶促氧化翻译后修饰 (PTM),已在数百种蛋白质中发现。然而,在大多数情况下,人们对半胱氨酸亚磺化的影响知之甚少。Cys-SO2- 具有独特的结构,具有长硫碳键和硫氧键,并且由于其孤对而在硫周围具有四面体几何结构。因此,Cys-SO2- 与蛋白质主链具有独特的潜在相互作用范围,可促进蛋白质结构变化。在此,使用核磁共振光谱、圆二色性、生物信息学和计算研究在模型肽和折叠蛋白中研究了半胱氨酸氧化为亚磺酸的结构效应。在 PDB 中,Cys-SO2- 比 Cys 更倾向于 α-螺旋。此外,Cys-SO2- 更常见于 φ > 0 的结构,包括多种类型的 β-转角。亚磺酸盐氧与相邻的 (i 或 i + 1) 酰胺氢形成氢键。超过一半的亚磺酸盐与相邻的酰胺至少有一个氢键,并且一些结构与两个相邻的酰胺都具有氢键。或者,硫或氧可以作为电子供体,与同一残基的主链羰基相互作用,如常出现的 S⋯CO 或 O⋯CO 距离低于它们在蛋白质中的范德华半径之和所示结构。在肽中,Cys-SO2- 有利于 N 末端的 α-螺旋结构,与螺旋偶极效应和主链氢键与亚磺酸盐促进 α-螺旋一致。Cys-SO2- 的聚脯氨酸 II 螺旋倾向仅略高于 Cys-SH,可能是由于来自多个侧链主链相互作用的竞争。Cys-SO2- 稳定 β-转角相对于 Cys-SH 的 i+1 位置。在蛋白质中,与 Cys-SH 相比,Cys-SO2- 可用的侧链-主链相互作用范围为由于半胱氨酸氧化为亚磺酸而导致的蛋白质结构和功能的潜在变化提供了基础。
更新日期:2019-12-27
中文翻译:

半胱氨酸亚磺酸的结构偏好:亚磺酸盐与肽骨架进行多种局部相互作用。
半胱氨酸亚磺酸 (Cys-SO2-) 是一种非酶促氧化翻译后修饰 (PTM),已在数百种蛋白质中发现。然而,在大多数情况下,人们对半胱氨酸亚磺化的影响知之甚少。Cys-SO2- 具有独特的结构,具有长硫碳键和硫氧键,并且由于其孤对而在硫周围具有四面体几何结构。因此,Cys-SO2- 与蛋白质主链具有独特的潜在相互作用范围,可促进蛋白质结构变化。在此,使用核磁共振光谱、圆二色性、生物信息学和计算研究在模型肽和折叠蛋白中研究了半胱氨酸氧化为亚磺酸的结构效应。在 PDB 中,Cys-SO2- 比 Cys 更倾向于 α-螺旋。此外,Cys-SO2- 更常见于 φ > 0 的结构,包括多种类型的 β-转角。亚磺酸盐氧与相邻的 (i 或 i + 1) 酰胺氢形成氢键。超过一半的亚磺酸盐与相邻的酰胺至少有一个氢键,并且一些结构与两个相邻的酰胺都具有氢键。或者,硫或氧可以作为电子供体,与同一残基的主链羰基相互作用,如常出现的 S⋯CO 或 O⋯CO 距离低于它们在蛋白质中的范德华半径之和所示结构。在肽中,Cys-SO2- 有利于 N 末端的 α-螺旋结构,与螺旋偶极效应和主链氢键与亚磺酸盐促进 α-螺旋一致。Cys-SO2- 的聚脯氨酸 II 螺旋倾向仅略高于 Cys-SH,可能是由于来自多个侧链主链相互作用的竞争。Cys-SO2- 稳定 β-转角相对于 Cys-SH 的 i+1 位置。在蛋白质中,与 Cys-SH 相比,Cys-SO2- 可用的侧链-主链相互作用范围为由于半胱氨酸氧化为亚磺酸而导致的蛋白质结构和功能的潜在变化提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号