当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amplified luminescence quenching effect upon binding of nitrogen doped carbon nanodots to transition metal ions.
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-21 , DOI: 10.1039/c9pp00420c J S Anjali Devi 1 , R S Aparna , R R Anjana , N S Vijila , J Jayakrishna , Sony George
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2020-01-21 , DOI: 10.1039/c9pp00420c J S Anjali Devi 1 , R S Aparna , R R Anjana , N S Vijila , J Jayakrishna , Sony George
Affiliation
|
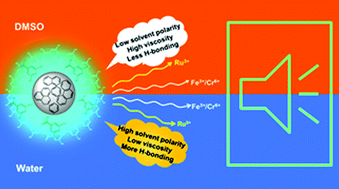
There is a significant drive to identify a unified emission mechanism hidden behind carbon nanodots (CDs) to attain reliable control over their photoluminescence properties. This issue is addressed here by investigating the fluorescence response of citric acid and urea-based nitrogen doped carbon nanodots (NCDs) towards transition metal ions in solutions of different polarities/viscosities/hydrogen bonding strengths. The photoluminescence from NCDs upon excitation at 400 nm is quenched by metal ions such as chromium(vi), ruthenium(iii) and iron(iii) in two different polar solvents, protic water and aprotic dimethylsulphoxide (DMSO). This amplified luminescence quenching in polar solutions showed significant static quenching contributions. The quenching phenomenon highly depends on the excitation wavelength and solvent environment. The fluorescence quenching sequence reveals that pyridinic nitrogen-bases have a dominant influence on J-like emissive aggregates of NCDs. Similarly, oxygen-containing functional groups play a significant role in constructing H-aggregates of NCDs. The most intense emission is contributed by the J-like assembly of H-aggregates.
中文翻译:

氮掺杂碳纳米点与过渡金属离子结合后的增强的发光猝灭作用。
有很大的动力来确定隐藏在碳纳米点(CD)后面的统一发射机制,以实现对其光致发光特性的可靠控制。通过研究柠檬酸和尿素基氮掺杂碳纳米点(NCD)对不同极性/粘度/氢键强度溶液中的过渡金属离子的荧光响应,可以解决此问题。在两种不同的极性溶剂,质子水和非质子二甲基亚砜(DMSO)中,金属离子(例如铬(vi),钌(iii)和铁(iii))淬灭了400 nm激发的NCD发出的光致发光。在极性溶液中这种放大的发光猝灭显示出显着的静态猝灭贡献。猝灭现象高度取决于激发波长和溶剂环境。荧光猝灭序列显示,吡啶氮基对NCD的J型发射聚集体具有主要影响。同样,含氧官能团在构建NCD的H聚集体中起重要作用。最强的发射是由H聚集体的类J组装引起的。
更新日期:2020-02-19
中文翻译:

氮掺杂碳纳米点与过渡金属离子结合后的增强的发光猝灭作用。
有很大的动力来确定隐藏在碳纳米点(CD)后面的统一发射机制,以实现对其光致发光特性的可靠控制。通过研究柠檬酸和尿素基氮掺杂碳纳米点(NCD)对不同极性/粘度/氢键强度溶液中的过渡金属离子的荧光响应,可以解决此问题。在两种不同的极性溶剂,质子水和非质子二甲基亚砜(DMSO)中,金属离子(例如铬(vi),钌(iii)和铁(iii))淬灭了400 nm激发的NCD发出的光致发光。在极性溶液中这种放大的发光猝灭显示出显着的静态猝灭贡献。猝灭现象高度取决于激发波长和溶剂环境。荧光猝灭序列显示,吡啶氮基对NCD的J型发射聚集体具有主要影响。同样,含氧官能团在构建NCD的H聚集体中起重要作用。最强的发射是由H聚集体的类J组装引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号