Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide interdigitation-induced twisted nanoribbons as chiral scaffolds for supramolecular nanozymes.
Nanoscale ( IF 5.8 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9nr09492j Shuxin Song 1 , Jingyu Wang 2 , Na Song 1 , Huixia Di 3 , Dingbin Liu 3 , Zhilin Yu 1
Nanoscale ( IF 5.8 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9nr09492j Shuxin Song 1 , Jingyu Wang 2 , Na Song 1 , Huixia Di 3 , Dingbin Liu 3 , Zhilin Yu 1
Affiliation
|
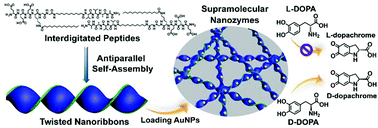
Establishing reliable strategies for rationally manipulating the organization of peptide building blocks and thereby precisely creating chiral nanostructures is challenging, while meaningful toward development of advanced functional materials. Here we report on a peptide-interdigitating mechanism for the reliable self-assembly of lipid-inspired amphiphiles (LIPIAs) into robust twisted nanoribbons by grafting domains to one alkyl tail of lipids as an extended element. Peptide interdigitation promoted the self-assembly of LIPIAs into twisted or flat nanoribbons driven by antiparallel or parallel β-sheet hydrogen bonds, respectively, strongly associated with the connecting direction of the incorporated domains. We found that the LIPIAs containing N-terminus-connected domains with either bulky or small side chain groups formed twisted nanoribbons in a broad pH range, thus implying a sequence- and pH-independent strategy for creation of robust chiral nanostructures. Integrating the resulting twisted nanoribbons with gold nanoparticles led to supramolecular nanozymes exhibiting the excellent catalytic activity and enantioselectivity of asymmetric oxidation of 3,4-dihyroxy-phenylalanine molecules. Our finding demonstrates that the peptide-interdigitating mechanism is a reliable strategy for precise creation of chiral nanostructures serving as chiral matrices for supramolecular nanozymes with improved catalytic performance, thus potentially paving the way towards advanced biomimetic systems resembling natural systems.
中文翻译:

肽指叉诱导的扭曲纳米带作为超分子纳米酶的手性支架。
建立合理的策略来合理地操纵肽结构单元的组织,从而精确地创建手性纳米结构是具有挑战性的,同时对开发先进的功能材料也很有意义。在这里,我们报道了通过将域嫁接到脂质的一个烷基尾部作为扩展元素,将脂质启发的两亲物(LIPIAs)可靠地自组装成坚固的扭曲纳米带的肽互指机制。肽相互交叉促进了LIPIA的自组装,分别由反平行或平行的β-sheet氢键驱动的扭曲或平坦的纳米带,与结合域的连接方向密切相关。我们发现,包含具有庞大或较小侧链基团的N末端连接结构域的LIPIAs在较宽的pH范围内形成了扭曲的纳米带,从而暗示了独立于序列和pH的策略来创建坚固的手性纳米结构。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。因此暗示了建立稳固的手性纳米结构的不依赖序列和pH的策略。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。因此暗示了建立稳固的手性纳米结构的不依赖序列和pH的策略。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。
更新日期:2020-01-09
中文翻译:

肽指叉诱导的扭曲纳米带作为超分子纳米酶的手性支架。
建立合理的策略来合理地操纵肽结构单元的组织,从而精确地创建手性纳米结构是具有挑战性的,同时对开发先进的功能材料也很有意义。在这里,我们报道了通过将域嫁接到脂质的一个烷基尾部作为扩展元素,将脂质启发的两亲物(LIPIAs)可靠地自组装成坚固的扭曲纳米带的肽互指机制。肽相互交叉促进了LIPIA的自组装,分别由反平行或平行的β-sheet氢键驱动的扭曲或平坦的纳米带,与结合域的连接方向密切相关。我们发现,包含具有庞大或较小侧链基团的N末端连接结构域的LIPIAs在较宽的pH范围内形成了扭曲的纳米带,从而暗示了独立于序列和pH的策略来创建坚固的手性纳米结构。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。因此暗示了建立稳固的手性纳米结构的不依赖序列和pH的策略。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。因此暗示了建立稳固的手性纳米结构的不依赖序列和pH的策略。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。将所得的扭曲纳米带与金纳米颗粒整合在一起,导致超分子纳米酶表现出出色的催化活性和3,4-二羟基-苯丙氨酸分子不对称氧化的对映选择性。我们的发现表明,肽叉指机制是精确创建手性纳米结构的可靠策略,该手性纳米结构用作具有改善的催化性能的超分子纳米酶的手性基质,因此有可能为类似于天然系统的高级仿生系统铺平道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号