当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibrium for Binary System of Isoamyl dl-Lactate and Isoamyl Alcohol at 25.0, 50.0, and 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.jced.9b00757 Jumei Xu 1 , Shating Li 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.jced.9b00757 Jumei Xu 1 , Shating Li 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Affiliation
|
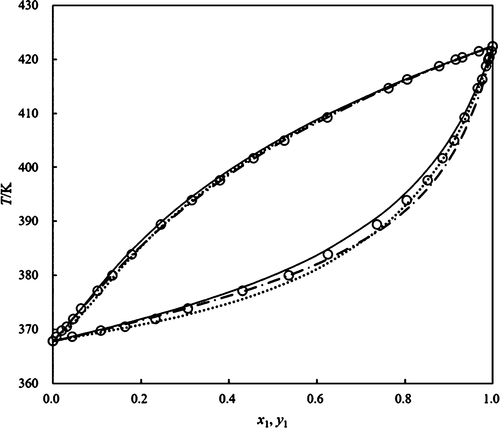
Isobaric vapor–liquid equilibrium (VLE) data for the binary system of isoamyl dl-lactate and isoamyl alcohol were measured at 25.0, 50.0, and 101.3 kPa in a modified Rose equilibrium still. The mixtures do not represent azeotropes. The determined experimental data were checked by Wisniak and van Ness tests to verify their thermodynamic consistency. Meanwhile, the Wilson, NRTL, and UNIQUAC models are adopted to correlate the VLE data, and the parameters of the three models were regressed. The UNIQUAC model showed the best fit to the experimental values relative to the Wilson and NRTL models, and it had the lowest root-mean-square deviations of equilibrium temperature (T) and mole fraction of the vapor phase (y1), with the value of 1.54 K and 0.0234, respectively.
中文翻译:

异戊酸dl-乳酸和异戊醇的二元体系的等压蒸气-液体平衡在25.0、50.0和101.3 kPa
在改良的Rose平衡状态下,异戊基dl-乳酸和异戊醇二元体系的等压气液平衡(VLE)数据分别为25.0、50.0和101.3 kPa。混合物不代表共沸物。通过Wisniak和van Ness测试检查确定的实验数据,以验证其热力学一致性。同时,采用Wilson,NRTL和UNIQUAC模型对VLE数据进行关联,并对这三个模型的参数进行回归。相对于Wilson和NRTL模型,UNIQUAC模型显示出与实验值的最佳拟合,并且平衡温度(T)和气相摩尔分数(y 1)的均方根偏差最小。),其值分别为1.54 K和0.0234。
更新日期:2019-12-27
中文翻译:

异戊酸dl-乳酸和异戊醇的二元体系的等压蒸气-液体平衡在25.0、50.0和101.3 kPa
在改良的Rose平衡状态下,异戊基dl-乳酸和异戊醇二元体系的等压气液平衡(VLE)数据分别为25.0、50.0和101.3 kPa。混合物不代表共沸物。通过Wisniak和van Ness测试检查确定的实验数据,以验证其热力学一致性。同时,采用Wilson,NRTL和UNIQUAC模型对VLE数据进行关联,并对这三个模型的参数进行回归。相对于Wilson和NRTL模型,UNIQUAC模型显示出与实验值的最佳拟合,并且平衡温度(T)和气相摩尔分数(y 1)的均方根偏差最小。),其值分别为1.54 K和0.0234。











































 京公网安备 11010802027423号
京公网安备 11010802027423号