当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quest for a Symmetric [C-F-C]+ Fluoronium Ion in Solution: A Winding Path to Ultimate Success.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.accounts.9b00554 Maxwell Gargiulo Holl 1 , Cody Ross Pitts 1 , Thomas Lectka 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.accounts.9b00554 Maxwell Gargiulo Holl 1 , Cody Ross Pitts 1 , Thomas Lectka 1
Affiliation
|
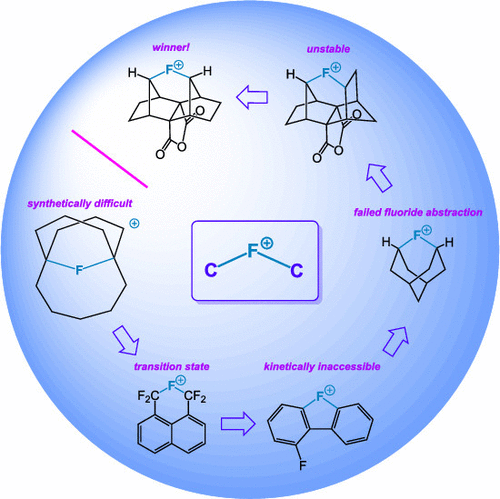
In this Account, we chronicle our tortuous but ultimately fruitful quest to synthesize a [C-F-C]+ fluoronium ion in solution, thus providing the last piece of the organic halonium ion puzzle. Inspiration for the project can be traced all the way back to the graduate career of the corresponding author, wherein the analogy between a [C-H-C]+ "hydrido" bridge and a hypothetical [C-F-C]+ bridge was first noted. The earliest attempt to construct a bicyclo[5.3.3]tridecane-based fluoronium ion (based on the analogous hydrido bridged cation) proved to be synthetically difficult. A subsequent attempt involving a 1,8-substituted naphthalene ring was theoretically naïve in retrospect, and it resulted in a classical benzylic carbocation instead. A biphenyl-based substrate, although computationally sound, proved to be kinetically untenable. At last, after some tweaking (including a dead-end detour into a fluoraadamantane skeleton), we finally achieved success with a highly rigid, semicage precursor based on the decahydro-1,4:5,8-dimethanonaphthalene system. This strained substrate possessed a triflate leaving group to enhance its solvolytic reactivity. Detailed isotopic labeling and kinetic studies supported the generation of a symmetrical [C-F-C]+ bridge; interesting solution behavior allowed the manipulation of the rate-determining step for solvolysis depending on solvent nucleophilicity. After initial generation as a transient intermediate, the fluoronium ion was later produced as a stable species in solution and was fully characterized by 19F, 1H, and 13C NMR, with the resultant species displaying evident Cssymmetry through coordination of a molecule of SbF5. This remarkable ion proved stable to -30 °C. We also address a disagreement surrounding the nomenclature of fluoronium ions in particular and its potential impact upon the naming of onium ions in general. We strove to highlight the dangers of confusing the arbitrary concept of calculated partial charge with IUPAC nomenclature. Finally, we discuss future directions, for example, the synthesis of a fluoronium ion in which fluorine resides within an aromatic ring.
中文翻译:

寻求解决方案中对称的[CFC] +氟离子:通往成功之路。
在这个帐户中,我们将曲折但最终富有成果的编年史编入序,以合成溶液中的[CFC] +氟离子,从而提供了有机ha离子难题的最后一部分。该项目的灵感可以追溯到相应作者的研究生生涯,其中首先提到了[CHC] +“氢”桥与假设的[CFC] +桥之间的类比。构造基于双环[5.3.3]十三烷的氟离子(基于类似的氢化桥联阳离子)的最早尝试被证明是合成困难的。从理论上讲,随后涉及1,8-取代的萘环的尝试在理论上是幼稚的,而是导致了经典的苄基碳正离子化。基于联苯的底物,尽管在计算上是合理的,但在动力学上却是站不住脚的。终于,经过一些调整(包括完全弯曲到氟金刚烷骨架上),我们终于成功使用基于十氢-1,4:5,8-二甲萘基萘体系的高刚性,半笼状前驱体获得了成功。该应变的底物具有三氟甲磺酸酯离去基团,以增强其溶剂分解反应性。详细的同位素标记和动力学研究支持对称[CFC] +桥的生成。有趣的溶液行为使得可以根据溶剂的亲核性对溶剂分解的速率确定步骤进行操作。在最初生成为过渡中间体之后,氟离子随后作为溶液中的稳定物种生成,并通过19F,1H和13C NMR进行了全面表征,所得物种通过SbF5分子的配位表现出明显的对称性。事实证明,这种出色的离子在-30°C下稳定。我们还解决了围绕氟离子命名法的分歧,尤其是它对洋葱离子命名的潜在影响。我们力求突出将IUPAC命名法混淆计算的部分费用的任意概念的危险。最后,我们讨论了未来的方向,例如,氟在芳环内的氟离子的合成。
更新日期:2019-12-27
中文翻译:

寻求解决方案中对称的[CFC] +氟离子:通往成功之路。
在这个帐户中,我们将曲折但最终富有成果的编年史编入序,以合成溶液中的[CFC] +氟离子,从而提供了有机ha离子难题的最后一部分。该项目的灵感可以追溯到相应作者的研究生生涯,其中首先提到了[CHC] +“氢”桥与假设的[CFC] +桥之间的类比。构造基于双环[5.3.3]十三烷的氟离子(基于类似的氢化桥联阳离子)的最早尝试被证明是合成困难的。从理论上讲,随后涉及1,8-取代的萘环的尝试在理论上是幼稚的,而是导致了经典的苄基碳正离子化。基于联苯的底物,尽管在计算上是合理的,但在动力学上却是站不住脚的。终于,经过一些调整(包括完全弯曲到氟金刚烷骨架上),我们终于成功使用基于十氢-1,4:5,8-二甲萘基萘体系的高刚性,半笼状前驱体获得了成功。该应变的底物具有三氟甲磺酸酯离去基团,以增强其溶剂分解反应性。详细的同位素标记和动力学研究支持对称[CFC] +桥的生成。有趣的溶液行为使得可以根据溶剂的亲核性对溶剂分解的速率确定步骤进行操作。在最初生成为过渡中间体之后,氟离子随后作为溶液中的稳定物种生成,并通过19F,1H和13C NMR进行了全面表征,所得物种通过SbF5分子的配位表现出明显的对称性。事实证明,这种出色的离子在-30°C下稳定。我们还解决了围绕氟离子命名法的分歧,尤其是它对洋葱离子命名的潜在影响。我们力求突出将IUPAC命名法混淆计算的部分费用的任意概念的危险。最后,我们讨论了未来的方向,例如,氟在芳环内的氟离子的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号