当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzyme Promiscuity versus Fidelity in Two Sesquiterpene Cyclases (TEAS versus ATAS)
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b05051 Fan Zhang 1 , Tianyue An 2 , Xiaowen Tang 1 , Jiachen Zi 2 , Hai-Bin Luo 1 , Ruibo Wu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-07 , DOI: 10.1021/acscatal.9b05051 Fan Zhang 1 , Tianyue An 2 , Xiaowen Tang 1 , Jiachen Zi 2 , Hai-Bin Luo 1 , Ruibo Wu 1
Affiliation
|
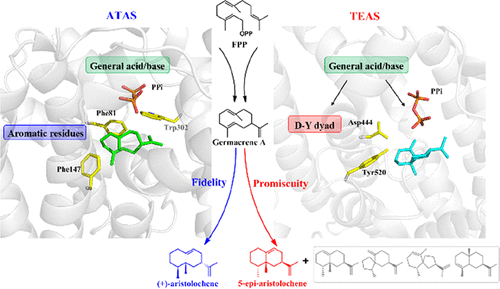
Catalytic promiscuity, as a modern and imperfect understanding concept in enzyme catalysis community, is prevailing in plant-derived sesquiterpene cyclases (FPPC) and highly related to the chemical diversity of sesquiterpenoid natural products. Both the Nicotiana tabacum 5-epi-aristolochene synthase (TEAS) and Aspergillus terreus aristolochene synthase (ATAS) belong to FPPC, involve the same reaction pathway, and yield their ultimate main product (aristolochene) with different stereochemistries. The catalytic promiscuity of TEAS and fidelity of ATAS have been observed in previous experimental studies, but the detailed catalytic mechanism is still not clear. Herein, by employing the quantum classical multiscale molecular dynamics simulations and site-directed mutagenesis experiments, the complete enzyme catalytic pathways from the substrate to aristolochene and various side products (in TEAS) are investigated and the important mechanism insights are included: (1) the PPi moiety (diphosphate group, released from the substrate) would further act as the general acid/base in both TEAS and ATAS enzyme catalysis, which likely plays a general role in FPPC. (2) The Asp444–Tyr520 dyad acts as an additional general acid/base residue pair to increase promiscuity in TEAS. (3) The enriched aromatic residues are essential for the catalytic fidelity of ATAS. Finally, we further discuss the three critical chemical control factors which are proposed to be responsible for the catalytic promiscuity and fidelity in most FPPC, that is, substrate folding mode, intermediate flexibility, and key residue, owing to the more or less plasticity of the active pocket in various FPPC.
中文翻译:

两种倍半萜烯环化酶中的酶混杂与保真度(TEAS与ATAS)
作为酶催化领域的现代且不完善的理解概念,催化混杂在植物衍生的倍半萜环化酶(FPPC)中盛行,并且与倍半萜类天然产物的化学多样性高度相关。两个烟草5-表-马兜铃烯合酶(TEAS)和土曲霉马兜铃属合成酶(ATAS)属于FPPC,涉及相同的反应途径,并产生具有不同立体化学的最终主产物(aristolochene)。在先前的实验研究中已经观察到TEAS的催化混杂性和ATAS的保真度,但是详细的催化机理仍不清楚。在本文中,通过使用量子经典多尺度分子动力学模拟和定点诱变实验,研究了从底物到马兜铃酶和各种副产物(在TEAS中)的完整酶催化途径,并包括重要的机理见解:(1) PPi部分(二磷酸基团,从底物释放)将进一步充当TEAS和ATAS酶催化中的一般酸/碱,这很可能在FPPC中起一般作用。(2)Asp444–Tyr520二聚体可作为额外的通用酸/碱残基对,以增加TEAS中的混杂性。(3)富集的芳族残基对于ATAS的催化保真度至关重要。最后,我们进一步讨论了三个关键的化学控制因素,这三个因素决定了大多数FPPC的催化混杂和保真度,即底物折叠模式,中间柔韧性和关键残留物,这归因于其或多或少的可塑性。各种FPPC中的活动口袋。
更新日期:2020-01-07
中文翻译:

两种倍半萜烯环化酶中的酶混杂与保真度(TEAS与ATAS)
作为酶催化领域的现代且不完善的理解概念,催化混杂在植物衍生的倍半萜环化酶(FPPC)中盛行,并且与倍半萜类天然产物的化学多样性高度相关。两个烟草5-表-马兜铃烯合酶(TEAS)和土曲霉马兜铃属合成酶(ATAS)属于FPPC,涉及相同的反应途径,并产生具有不同立体化学的最终主产物(aristolochene)。在先前的实验研究中已经观察到TEAS的催化混杂性和ATAS的保真度,但是详细的催化机理仍不清楚。在本文中,通过使用量子经典多尺度分子动力学模拟和定点诱变实验,研究了从底物到马兜铃酶和各种副产物(在TEAS中)的完整酶催化途径,并包括重要的机理见解:(1) PPi部分(二磷酸基团,从底物释放)将进一步充当TEAS和ATAS酶催化中的一般酸/碱,这很可能在FPPC中起一般作用。(2)Asp444–Tyr520二聚体可作为额外的通用酸/碱残基对,以增加TEAS中的混杂性。(3)富集的芳族残基对于ATAS的催化保真度至关重要。最后,我们进一步讨论了三个关键的化学控制因素,这三个因素决定了大多数FPPC的催化混杂和保真度,即底物折叠模式,中间柔韧性和关键残留物,这归因于其或多或少的可塑性。各种FPPC中的活动口袋。











































 京公网安备 11010802027423号
京公网安备 11010802027423号