当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of a Small Molecule Probe of Rpn-6, an Essential Subunit of the 26S Proteasome.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-10 , DOI: 10.1021/acschembio.9b01019 Wenzhi Tian 1 , Darci J Trader 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-10 , DOI: 10.1021/acschembio.9b01019 Wenzhi Tian 1 , Darci J Trader 1
Affiliation
|
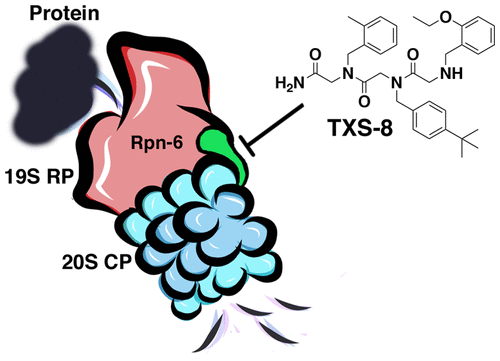
A considerable number of essential cellular proteins have no catalytic activity and serve instead as structural components to aid in assembling protein complexes. For example, the assembly and function of the 26S proteasome, the major enzymatic complex necessary for ubiquitin-dependent protein degradation, require a number of essential protein contacts to associate the 19S regulatory particle with the 20S core particle. Previously, small molecule inhibitors of the active sites of the 20S core particle have been developed, but the activity of the 26S proteasome could also be altered via the disruption of its assembly. We were interested in discovering a small molecule binder of Rpn-6, as it is among several essential proteins that facilitate 26S assembly, which could be used to further our understanding of the association of the 19S regulatory particle with the 20S core particle. Additionally, we were interested in whether a small molecule-Rpn-6 interaction could potentially be cytotoxic to cancer cells that rely heavily on proteasome activity for survival. A workflow for utilizing a one-bead, one-compound library and a thermal shift assay was developed to discover such a molecule. TXS-8, our lead hit, was discovered to have a low micromolar binding affinity for Rpn-6 as well as very limited binding to other proteins. The cytotoxicity of TXS-8 was evaluated in several cell lines, revealing increased cytotoxicity to hematological cancers. Discovery of this peptoid binder of Rpn-6 provides the initial evidence that Rpn-6 could be a druggable target to affect protein degradation and serves as a primary scaffold from which to design more potent binders. We suspect that Rpn-6 could have additional essential roles beyond that of a molecular clamp of the proteasome to help hematological cancer cells survive and that TXS-8 can serve as a useful tool for further elucidating its roles.
中文翻译:

Rpn-6的小分子探针的发现,Rpn-6是26S蛋白酶体的必需亚基。
相当数量的必需细胞蛋白没有催化活性,而是充当有助于组装蛋白复合物的结构成分。例如,26S蛋白酶体(泛素依赖性蛋白降解所必需的主要酶复合物)的组装和功能需要大量必需的蛋白接触才能使19S调控颗粒与20S核心颗粒缔合。以前,已经开发了20S核心颗粒活性位点的小分子抑制剂,但是26S蛋白酶体的活性也可以通过破坏其组装而改变。我们感兴趣的是发现Rpn-6的小分子结合剂,因为它是促进26S组装的几种必需蛋白之一,可以用来进一步了解19S调节颗粒与20S核心颗粒的结合。另外,我们对小分子-Rpn-6相互作用是否可能对严重依赖蛋白酶体活性生存的癌细胞具有细胞毒性感兴趣。开发了一种利用单珠单化合物文库和热位移测定法的工作流程来发现这种分子。发现我们的主打产品TXS-8对Rpn-6的微摩尔结合亲和力低,与其他蛋白质的结合非常有限。在几种细胞系中评估了TXS-8的细胞毒性,揭示了其对血液系统癌症的细胞毒性增加。Rpn-6的类肽结合剂的发现提供了最初的证据,证明Rpn-6可能是影响蛋白质降解的可药物化靶标,并用作设计更有效结合剂的主要支架。我们怀疑Rpn-6可能具有蛋白酶体分子钳之外的其他重要作用,以帮助血液癌细胞存活,并且TXS-8可以用作进一步阐明其作用的有用工具。
更新日期:2020-01-10
中文翻译:

Rpn-6的小分子探针的发现,Rpn-6是26S蛋白酶体的必需亚基。
相当数量的必需细胞蛋白没有催化活性,而是充当有助于组装蛋白复合物的结构成分。例如,26S蛋白酶体(泛素依赖性蛋白降解所必需的主要酶复合物)的组装和功能需要大量必需的蛋白接触才能使19S调控颗粒与20S核心颗粒缔合。以前,已经开发了20S核心颗粒活性位点的小分子抑制剂,但是26S蛋白酶体的活性也可以通过破坏其组装而改变。我们感兴趣的是发现Rpn-6的小分子结合剂,因为它是促进26S组装的几种必需蛋白之一,可以用来进一步了解19S调节颗粒与20S核心颗粒的结合。另外,我们对小分子-Rpn-6相互作用是否可能对严重依赖蛋白酶体活性生存的癌细胞具有细胞毒性感兴趣。开发了一种利用单珠单化合物文库和热位移测定法的工作流程来发现这种分子。发现我们的主打产品TXS-8对Rpn-6的微摩尔结合亲和力低,与其他蛋白质的结合非常有限。在几种细胞系中评估了TXS-8的细胞毒性,揭示了其对血液系统癌症的细胞毒性增加。Rpn-6的类肽结合剂的发现提供了最初的证据,证明Rpn-6可能是影响蛋白质降解的可药物化靶标,并用作设计更有效结合剂的主要支架。我们怀疑Rpn-6可能具有蛋白酶体分子钳之外的其他重要作用,以帮助血液癌细胞存活,并且TXS-8可以用作进一步阐明其作用的有用工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号