当前位置:
X-MOL 学术
›
Fuel Process. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism investigation on the formation of olefins and paraffin from the thermochemical catalytic conversion of triglycerides catalyzed by alkali metal catalysts
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fuproc.2019.106312 Feng Long , Xiaolei Zhang , Xincheng Cao , Qiaolong Zhai , Yaoguang Song , Fei Wang , Jianchun Jiang , Junming Xu
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.fuproc.2019.106312 Feng Long , Xiaolei Zhang , Xincheng Cao , Qiaolong Zhai , Yaoguang Song , Fei Wang , Jianchun Jiang , Junming Xu
|
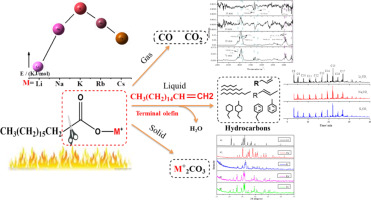
Abstract Triglycerides are a promising biomass feedstock that can be used for production of organic hydrocarbons including long-chain olefins and paraffin. The challenge for this production process lies on the lack of a clear mechanism of the conversion process. In this work, the conversion mechanism from triglycerides to olefins and paraffin using alkali metal catalysts was investigated adopting both computational calculations using density functional theory and experimental studies. The bond dissociation energies of the main bonds were calculated, especially for the α carbon‑carbon bond, which leads to effective removal of carboxyl groups during the thermochemical conversion process. The dynamic behavior of triglycerides catalyzed by alkali metal catalysts was also investigated using thermogravimetric analysis, which found that Li ion has lowest activation energy below 200 kJ/mol when compared with the other alkali ions studied. The catalytic conversion mechanism was proposed in this work based on the results obtained from TG-IR, GC, GC–MS and XRD analyses. The O atoms are removed in the form of CO, CO2 and H2O, product M + O and M+, which generates M2CO3. A more detailed mechanism has been proposed in this paper, which has significance toward guiding the cleavage of triglycerides to produce long‑carbon-chain terminal olefins and normal paraffin.
中文翻译:

碱金属催化剂热化学催化转化甘油三酯生成烯烃和石蜡的机理研究
摘要 甘油三酯是一种很有前景的生物质原料,可用于生产包括长链烯烃和石蜡在内的有机烃。这种生产过程的挑战在于缺乏明确的转化过程机制。在这项工作中,通过使用密度泛函理论的计算计算和实验研究,研究了使用碱金属催化剂从甘油三酯到烯烃和链烷烃的转化机理。计算了主要键的键解离能,特别是对于 α 碳-碳键,这导致在热化学转化过程中有效去除羧基。还使用热重分析研究了碱金属催化剂催化的甘油三酯的动态行为,发现与研究的其他碱离子相比,锂离子的活化能最低,低于 200 kJ/mol。在这项工作中,基于 TG-IR、GC、GC-MS 和 XRD 分析的结果,提出了催化转化机制。O 原子以 CO、CO2 和 H2O、产物 M + O 和 M+ 的形式被去除,从而生成 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。产生 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。产生 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。
更新日期:2020-04-01
中文翻译:

碱金属催化剂热化学催化转化甘油三酯生成烯烃和石蜡的机理研究
摘要 甘油三酯是一种很有前景的生物质原料,可用于生产包括长链烯烃和石蜡在内的有机烃。这种生产过程的挑战在于缺乏明确的转化过程机制。在这项工作中,通过使用密度泛函理论的计算计算和实验研究,研究了使用碱金属催化剂从甘油三酯到烯烃和链烷烃的转化机理。计算了主要键的键解离能,特别是对于 α 碳-碳键,这导致在热化学转化过程中有效去除羧基。还使用热重分析研究了碱金属催化剂催化的甘油三酯的动态行为,发现与研究的其他碱离子相比,锂离子的活化能最低,低于 200 kJ/mol。在这项工作中,基于 TG-IR、GC、GC-MS 和 XRD 分析的结果,提出了催化转化机制。O 原子以 CO、CO2 和 H2O、产物 M + O 和 M+ 的形式被去除,从而生成 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。产生 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。产生 M2CO3。该论文提出了更详细的机理,对指导甘油三酯裂解产生长碳链末端烯烃和正构烷烃具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号